Ionic Product versus Solubility Product
In this video created by Chemistry Guru, Singapore's leading JC Chemistry tuition centre, we want to discuss the concept of Ionic Product versus Solubility Product.
Let's use the dissociation of a sparingly soluble salt calcium hydroxide as an example.
Solubility Product
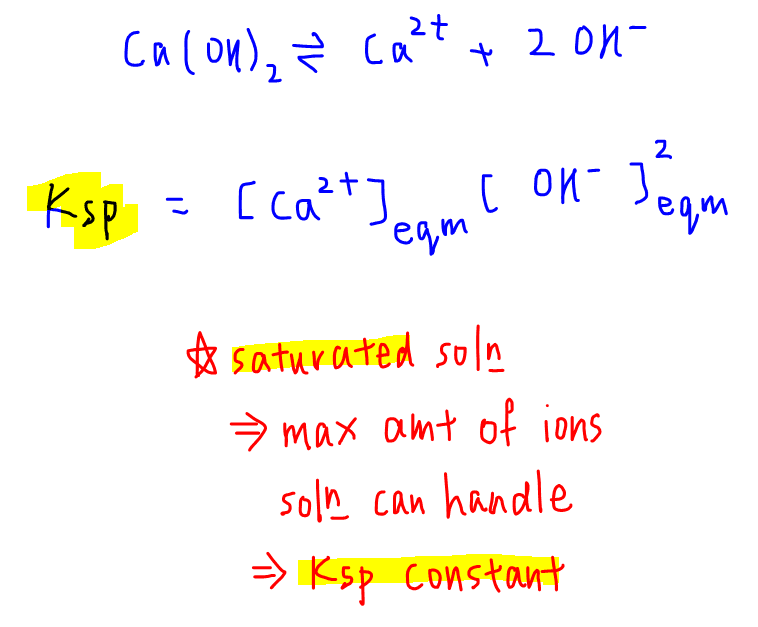
Based on the dissociation of calcium hydroxide, solubility product is given as:
Ksp = [Ca2+][OH-]2
Equilibrium constant Ksp describes the concentration of ions for a saturated solution, hence the concentration of Ca2+ and OH- are at their maximum and equilibrium value.
This means Ksp measures the maximum amount of ions that the solution can handle or dissolve.
Since Ksp is an equilibrium constant, it is only affected by changes in temperature, so will be a fixed value at constant temperature.
Ionic Product
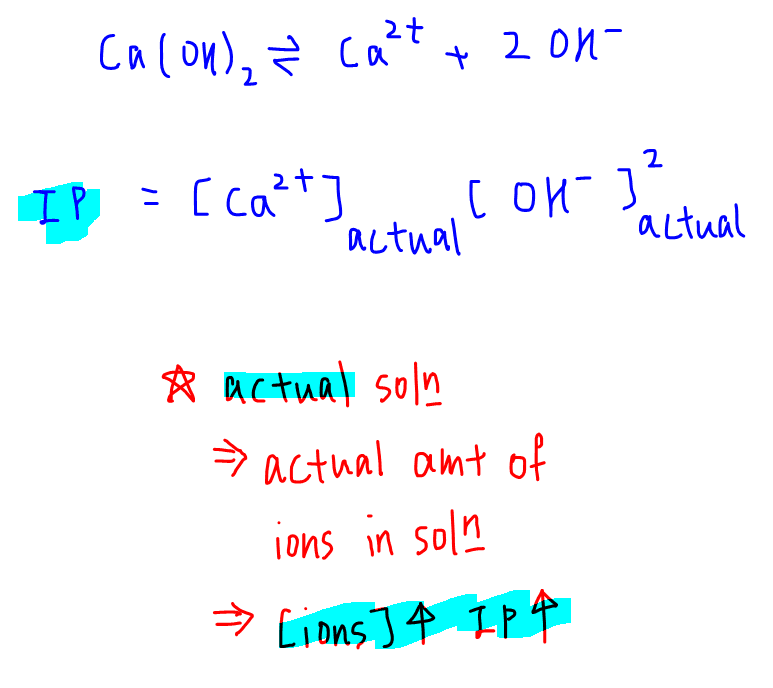
Interestingly ionic product looks exactly the same as solubility product:
IP = [Ca2+][OH-]2
However the concentration of ions are for any solution so the solution can be diluted, saturated or supersaturated.
Since ionic product measures the actual amount of ions in solution, it will vary depending on the concentration of ions dissolved.
Ionic Product versus Solubility Product
To visualise the relationship between IP and Ksp, we can see IP as the number line, which increases as concentration of ion increases, and Ksp as a specific point along this number line.
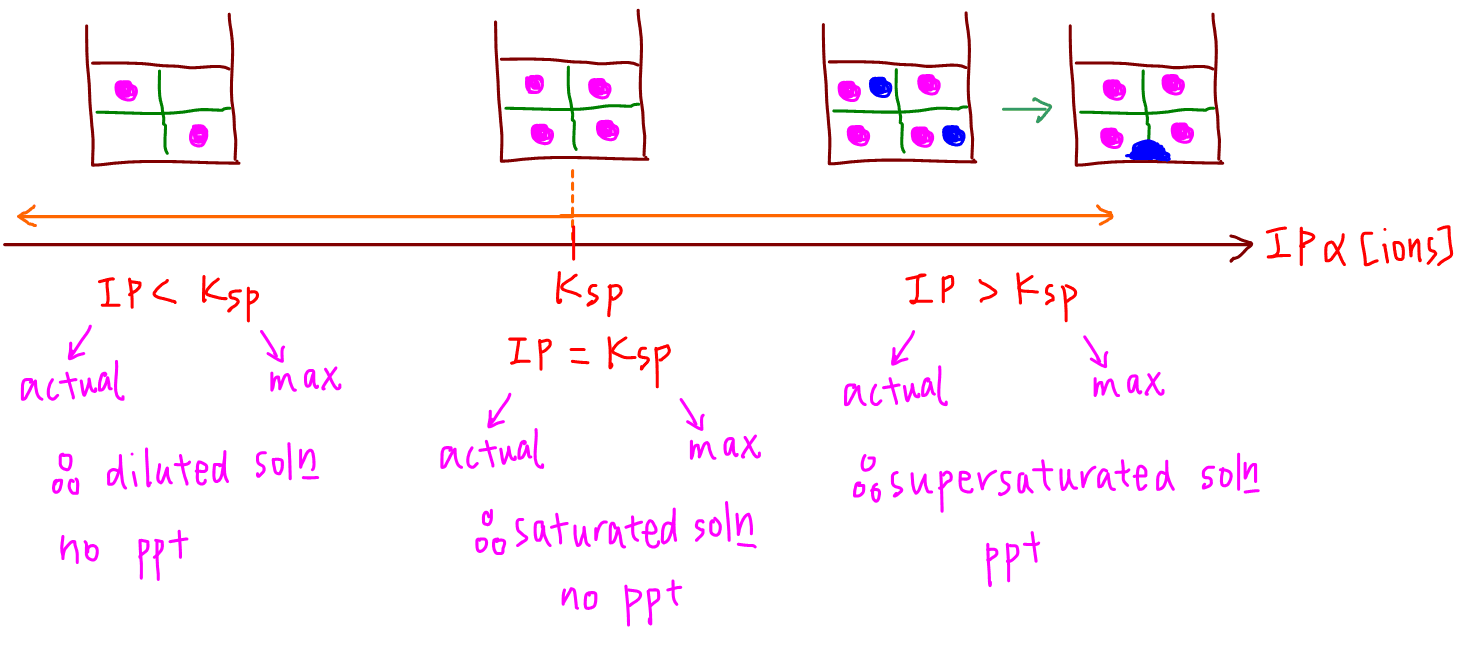
1. IP = Ksp
When actual amount of ions equal to maximum amount of ions, the solution is saturated but there is no precipitation.
Any addition of ions will result in precipitation since the saturated solution can no longer dissolve any more ions.
2. IP < Ksp
When actual amount of ions is less than maximum amount of ions, the solution is diluted and there is no precipitation.
We will expect more salt to dissolve when added since there are still available slots in the diluted solution to accommodate these additional ions.
3. IP > Ksp
When actual amount of ions is more than maximum amount of ions, the solution is supersaturated and is hanging on to more ions than it can keep in solution.
This usually happens when 2 solutions containing separate sources of cation and anion are mixed together.
A supersaturated solution doesn't stay supersaturated and it has to precipitate out the excess ions that it cannot handle in solution.
The resultant outcome will be a saturated solution and precipitate.
For the detailed comparison between ionic product and solubility product, check out this video!
Topic: Solubility Product, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
A Level H2 Chemistry Video Lessons
Chemistry Guru, Singapore's reputable JC Chemistry tuition centre, has a huge collection of short video lessons that targets important H2 Chemistry concepts and common questions.
Join my 19,000 subscribers on my YouTube Channel for new video lessons every week!
Follow me on Instagram for H2 Chemistry videos and (not so funny) memes!
You can also view other A Level H2 Chemistry videos here at my website.
Need an experienced tutor to make Chemistry simpler for you?
My weekly classes in Singapore are ideal for students who prefer a more structured program.
Build a strong foundation and ace your exams!
Sign up now for a trial lesson at $50only!
Check out our Chemistry tuition class timing and topics covered for our popular JC1 Classes and JC2 Classes at Bishan, Singapore.
Online lessons are also available! Learn H2 Chemistry anytime, anywhere at 50% of the cost of conventional class tuition.
Find out more information about our online tuition.
