IUPAC Nomenclature for Organic Compounds - Organic Chemistry
In this video we want to name the following organic compounds using the IUPAC Nomenclature system.
If you have learnt how to name organic compounds already, test yourself to see if you can name them correctly before going through the video!

Compound 1

IUPAC Nomenclature for organic compounds can be determined using the following steps:
1. Identify the most important functional group which is the acid group in this case.
2. Find the longest carbon chain that contains the functional group. For this compound the longest carbon chain is 4 carbon (butane)
3. Number the carbon chain giving priority to the functional group. So acid carbon will be assigned as number one, the iodo group assigned to carbon-2 and the 2 methyl groups to carbon-3.
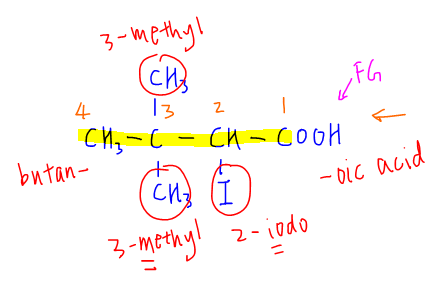
4. To name the compound, substituents will be placed first, followed by the carbon chain and finally the functional group.
Substituents are arranged in alphabetical order. If there are more than 1 of the same substituent, we can use di-, tri-, tetra- to represent the quantity of that substituent.
Finally we can combine everything together to name this organic compound:

Compound 2

The rules are essentially the same, but when there are no functional groups then priority will be given to the substituents when we number the carbon chain.
If there are more than 1 substituent, the sum of positions of all the substituents must be as small as possible.
This usually requires us to number the carbon chain from both ends, assign the numbers to the substituents and work out which side gives us the smallest sum.
We can simplify this by splitting the carbon chain into half, and count from the side where there are more groups so the smaller number will be assigned to the substituents and sum of the positions will naturally be smaller.

Again we arrange the substituents by alphabetical order to give the name of this compound.

Compound 3

When we have 2 substituents attached to a cycloalkane something interesting will happen.
One of these substituents should be assigned as position 1 so the sum of the positions will be smallest.
However the other substituent will then be assigned as position 3, which then gives us the outcome where the sum of positions will be exactly the same.

In this case we give priority to the first cited substituent, which is ethyl group for this example, so the name of the compound will be as follows:

For the detailed step-by-step discussion on how to name organic compounds using the IUPAC nomenclature system, check out this video!
Topic: Introduction to Organic Chemistry, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's leading JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online chemistry classes!
