Markovnikov Rule and Predicting Alkene Major Product
When an asymmetrical reactant such as HBr, HCl and H2O is added to an asymmetrical alkene, two possible products can be formed.
Markovnikov Rule, which states that hydrogen will be added to the carbon with more hydrogen, can be used to predict the major product of this reaction.
Applying Markovnikov Rule
Take for instance this alkene:
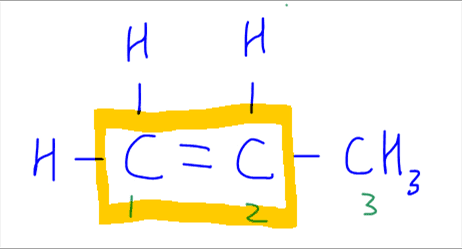
We notice that the alkene is asymmetrical as carbon-1 and carbon-2 are bonded to different groups.
Therefore if we add HBr to this alkene, 2 possible products can be formed.
Carbon-1 is bonded to 2 hydrogen, while carbon-2 is bonded to 1 hydrogen only.
Hence according to Markovnikov Rule, when hydrogen is added to the carbon with more hydrogen, we will get the major product.
This means that when hydrogen is added to carbon-1, which has more hydrogen, and bromine is added to carbon-2, the product 2-bromopropane will be the major product.
Conversely when hydrogen is added to carbon-2, which has less hydrogen, and bromine is added to carbon-1, the product 1-bromopropane will be the minor product.

Explaining Markovnikov Rule using Stability of Carbocations
Let's explain Markovnikov Rule by discussing the electrophilic addition mechanism of alkene with HBr.
In the first step, electron rich alkene will attack hydrogen of HBr which is partial positive charge. Two possible intermediates can be formed as the alkene is asymmetrical.

For the structure on the left: when hydrogen is added to carbon-1 with more hydrogen, the carbocation intermediate (on carbon-2) formed is bonded to 2 electron donating alkyl groups.
More electron donating groups will stabilise the carbocation to a greater extent.
Hence it is more stable, more likely formed and eventually becomes the major product.
For the structure on the right: when hydrogen is added to carbon-2 with less hydrogen, the carbocation intermediate (on carbon-1) formed is bonded to only 1 electron donating alkyl group.
Less electron donating groups will stabilise the carbocation to a smaller extent.
Hence it is less stable, less likely formed and becomes the minor product.
Check out this video lesson to learn how to determine major product for alkene addition reactions using Markovnikov Rule, and learn how to compare stability of carbocations!
Topic: Alkenes, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
A Level H2 Chemistry Video Lessons
Chemistry Guru, Singapore's reputable JC Chemistry tuition centre, has a huge collection of short video lessons that targets important H2 Chemistry concepts and common questions.
Join my 19,000 subscribers on my YouTube Channel for new video lessons every week!
Follow me on Instagram for H2 Chemistry videos and (not so funny) memes!
You can also view other A Level H2 Chemistry videos here at my website.
Need an experienced tutor to make Chemistry simpler for you?
My weekly classes in Singapore are ideal for students who prefer a more structured program.
Build a strong foundation and ace your exams!
Sign up now for a trial lesson at $50only!
Check out our Chemistry tuition class timing and topics covered for our popular JC1 Classes and JC2 Classes at Bishan, Singapore.
Online lessons are also available! Learn H2 Chemistry anytime, anywhere at 50% of the cost of conventional class tuition.
Find out more information about our online tuition.
