Melting Point of Period 3 Elements
In Periodicity we need to explain the trend in melting point for Period 3 elements.
Let Chemistry Guru, Singapore's top JC Chemistry tuition centre, guide you through the melting point trend first.
There are a few points to note:
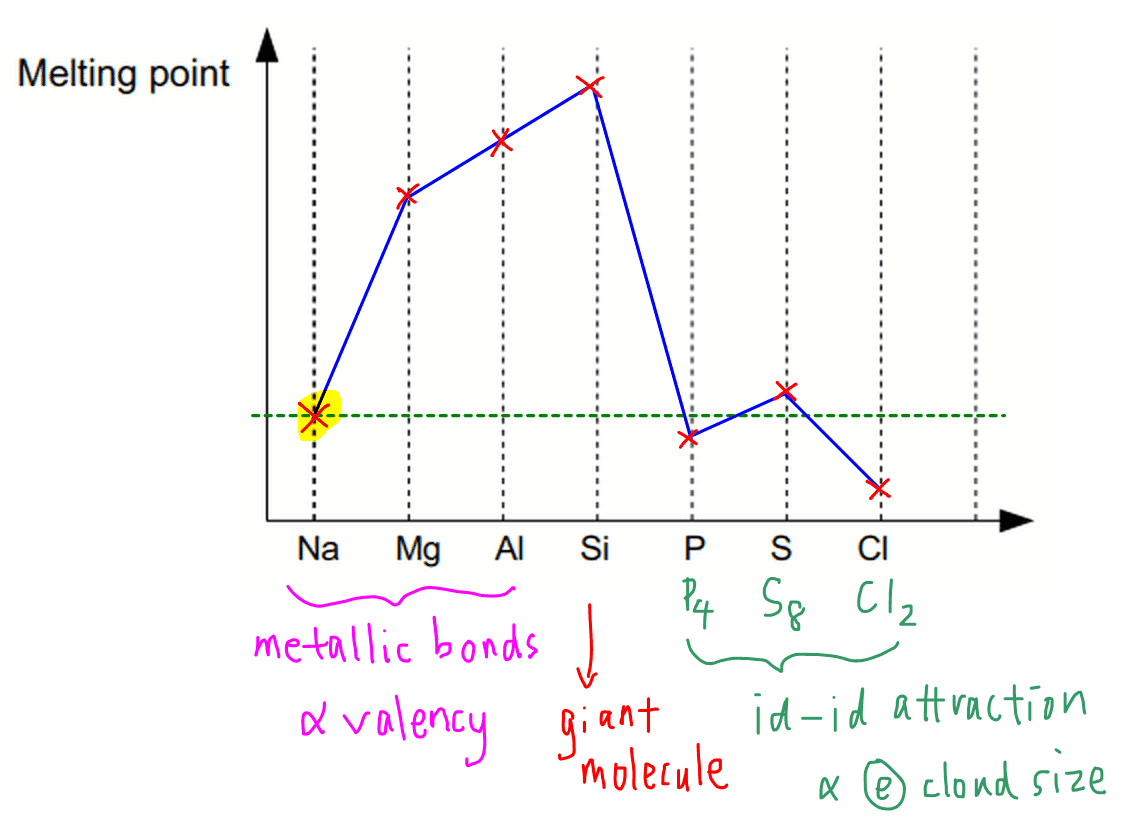
1. Melting point increases for metals Na, Mg and Al
Strength of metallic bonds is related to valency.
Across the period the valency increases (from valency 1 in sodium to valency 3 in aluminium) so the metal atoms can delocalise more electrons to form more positively charged cations and a bigger sea of delocalised electrons.
Therefore metallic bond becomes stronger and melting point increases from sodium to aluminium.
In fact the metallic bond in sodium and other Group 1 metals is so weak that the melting point of sodium is unusually low and falls within the region of simple molecules.
2. High melting point for Si
Silicon is a macromolecule or giant molecule with strong and extensive covalent bonds.
Therefore a lot of energy is required to overcome these covalent bonds and melting point for silicon is very high.
The melting point of Si is the highest in Period 3 elements but do take note this doesn't mean all giant molecules have higher melting points than all metals.
In Chemical Bonding we treat metallic bond, ionic bond and covalent bond as strong bonds hence melting points of metals, ionic compounds and giant molecules are all considered high.
In general we do not compare melting points between metals, ionic compounds and giant molecules.
3. Melting point for non-metals decrease in order S8, P4, Cl2
Phosphorus, sulphur and chlorine exist as simple molecules with molecular formula P4, S8 and Cl2 respectively.
Since they are non-polar, dominant intermolecular forces of attraction will be instantaneous dipole-induced dipole attraction, or dispersion forces or Van der Waals forces.
The larger and more polarisable the electron cloud, the stronger the id-id attraction between molecules hence the higher the melting point.
Since the molecular mass and electron cloud size is in the order S8 > P4 > Cl2, the melting point trend will be in that same order.
We can use the melting point of sodium as a benchmark for melting points of non-metals:
- P4 slightly below Na
- S8 slightly above Na
- Cl2 much lower than Na
Topic: Periodicity, Inorganic Chemistry, A Level Chemistry, Singapore
Back to other previous Inorganic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's renowned A Level Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online tuition classes!
