Deduce Optical Activity of Meso Compound
Let's compare these 2 compounds (A and B) and deduce which is optically inactive.
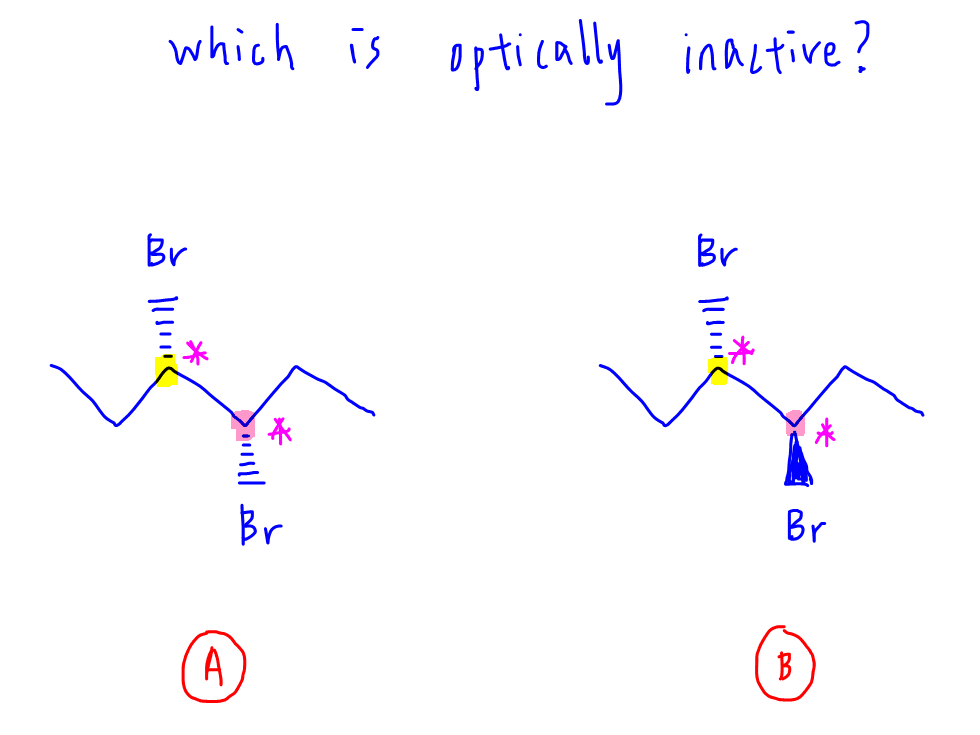
Each compound contains 2 chiral carbons.
A chiral carbon is a saturated sp3 hybridised carbon bonded to 4 different groups.
The mirror images of a compound with chiral carbon are non-superimposable hence they are isomers of each other.
We call these optical isomers or enantiomers.
Check out this video lesson to learn more about optical isomerism and other types of stereoisomerism.
What is interesting about compounds A and B is each chiral carbon is bonded to the same 4 groups:
- bromine
- hydrogen
- ethyl group CH2CH3
- the other chiral carbon
This means some of the optical isomers will have mirror symmetry which will cancel its optical activity.
In order to visualise and compare the chiral carbons better, let's apply the following transformations.
1. Convert ethyl group CH2CH3 to Et
The long carbon chain is distracting and since both carbons in ethyl group are non-chiral, we can just replace CH2CH3 with Et without affecting the stereochemistry of the compound.
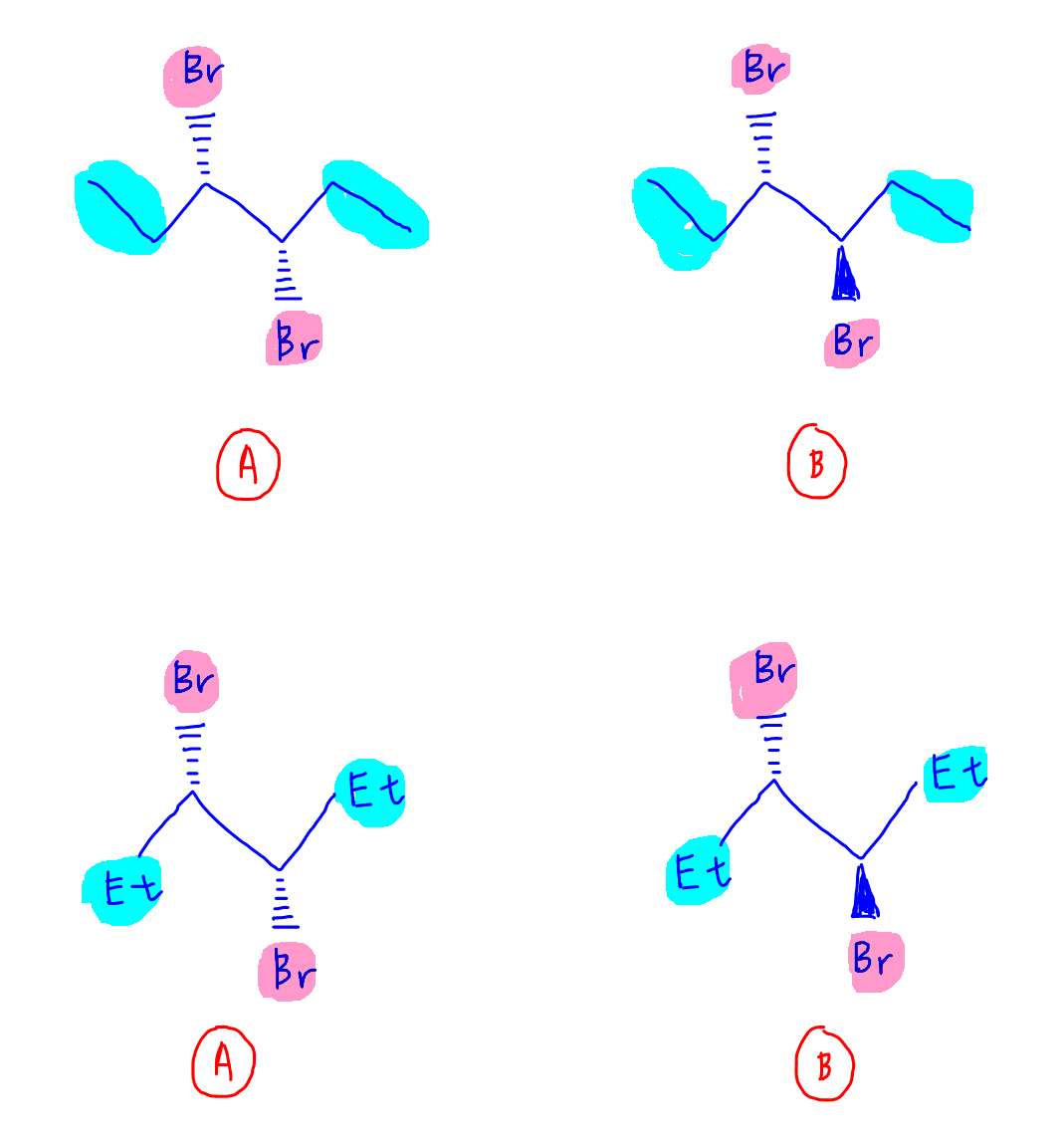
2. Rotate compound slightly
It will be easier to compare the compounds if the C-C bond for both chiral carbons is horizontal.
So let's rotate the compound slightly.
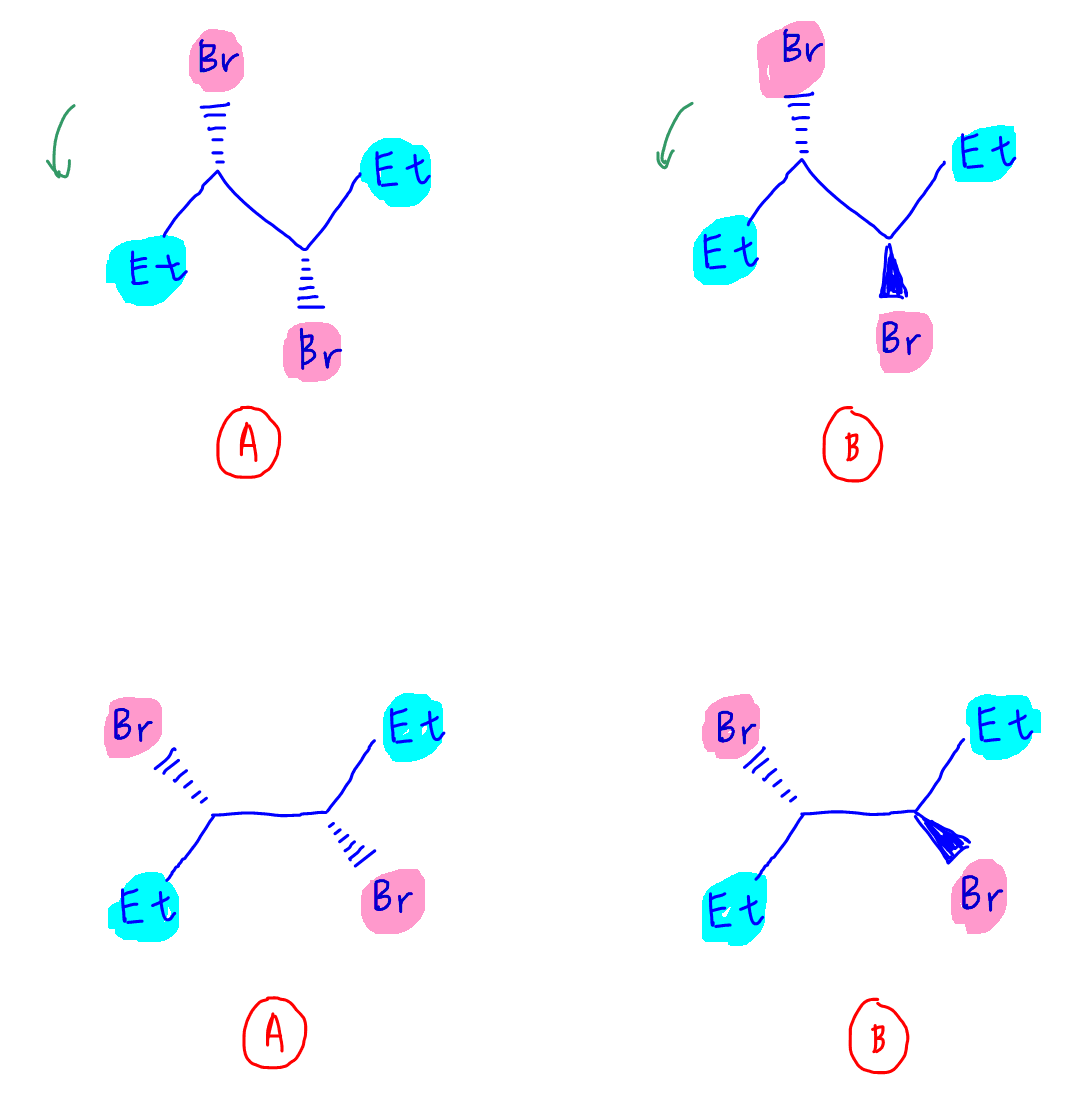
3. Rotate C-C bond by 180 degrees
We want both ethyl groups to point down to find the mirror plane easily so let's rotate the C-C bond by 180 degrees.
Notice the orientation of Br group will change due to this rotation:
- Br pointing towards you before rotation will be pointing away from you after rotation
- Br pointing away from you before rotation will be pointing towards you after rotation
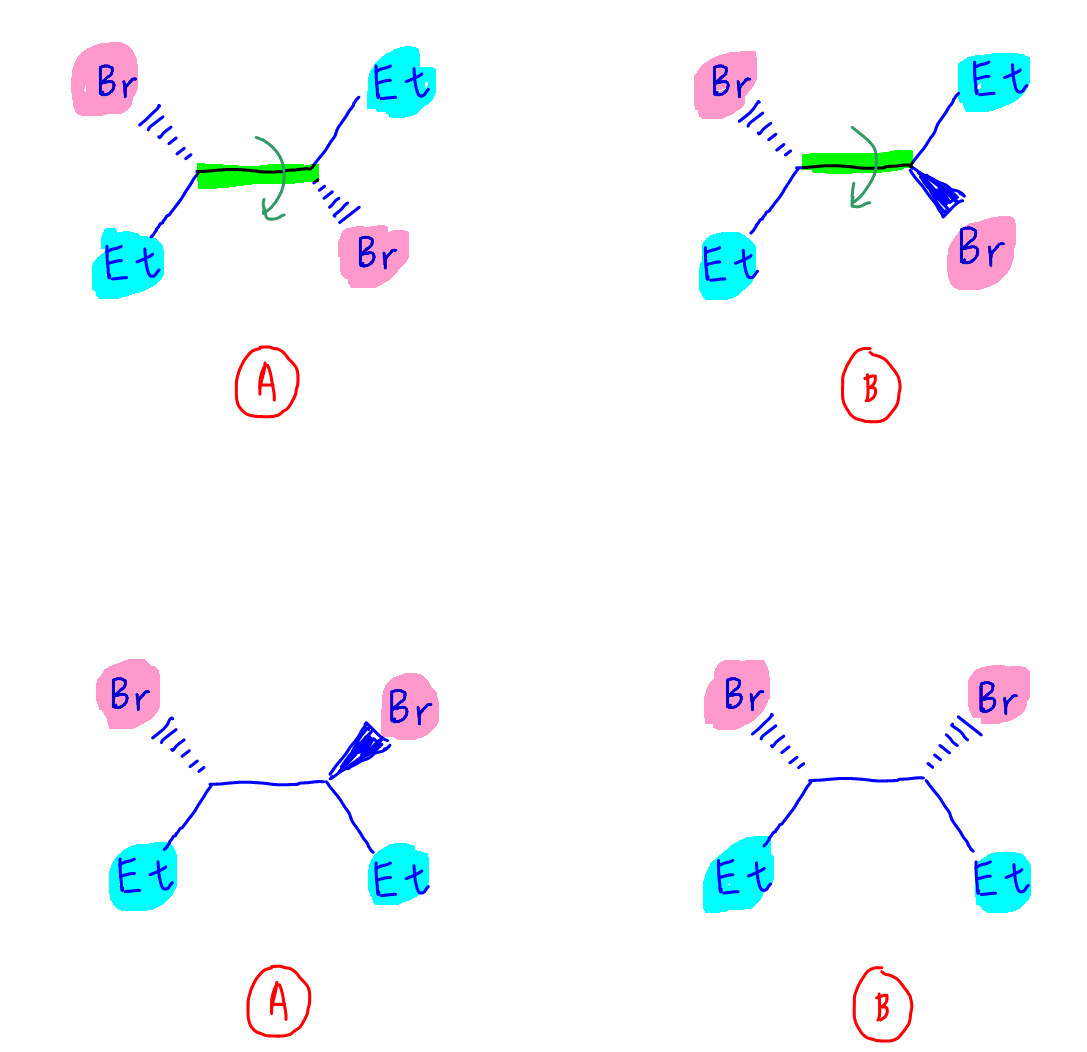
4. Find mirror plane
We can now compare the 2 compounds.
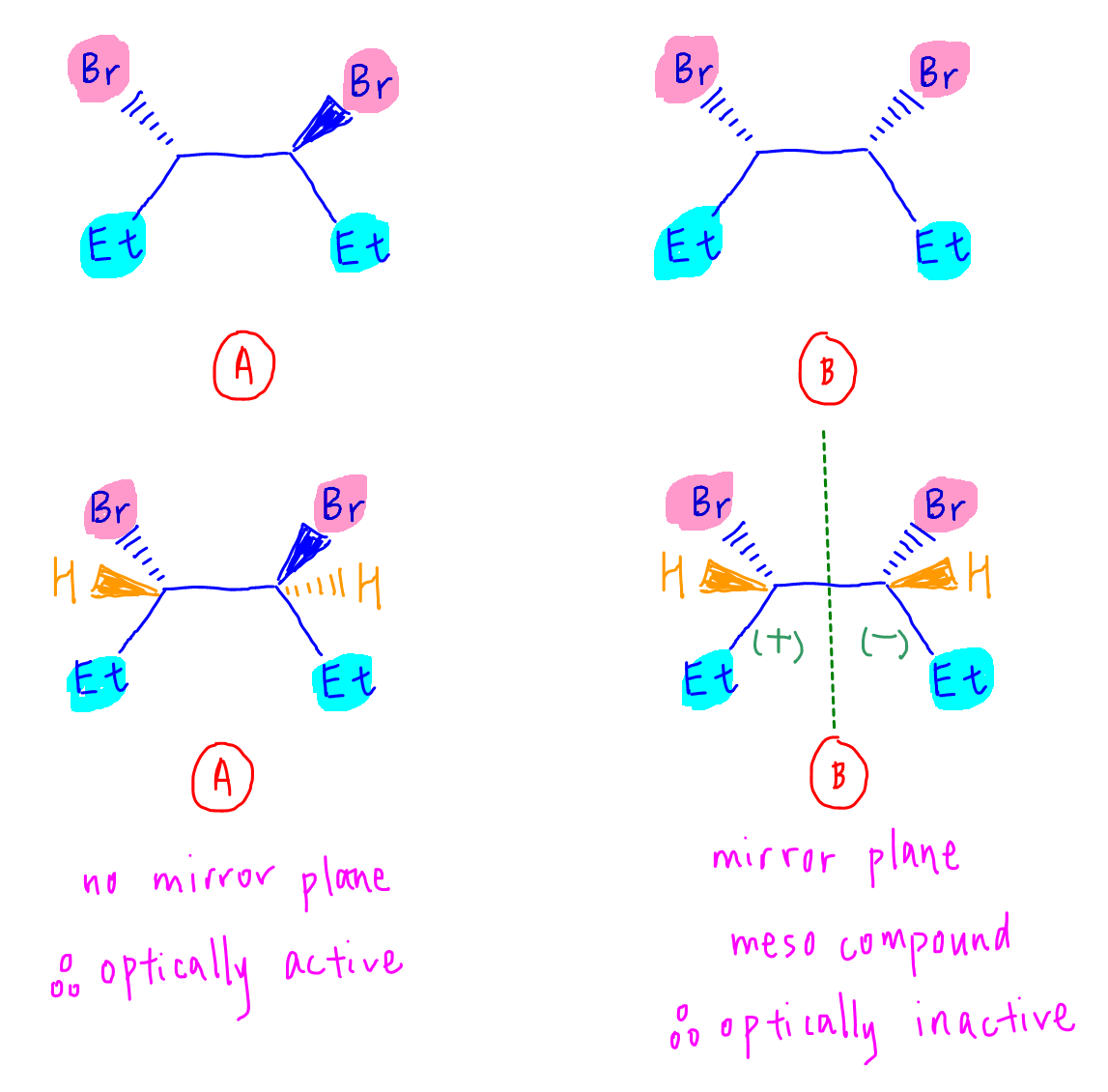
Compound A has no mirror plane so it will still be asymmetrical and optically active.
Compound B has a mirror plane between both chiral carbons.
The optical activities of both chiral carbons will cancel out.
Therefore compound B will be optically inactive.
Compound B is an example of a meso compound, where chiral carbons are present but the compound is optically inactive due to a mirror plane within the compound.
Topic: Intro to Organic Chemistry, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's esteemed H2 Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
