Mole Concept Worked Example - Determining Alcohol J
In this video created by Chemistry Guru, Singapore's leading JC Chemistry tuition centre, we have an interesting mole concept question where the molecular formula for alcohol J is unknown and given as CxHyOH and we are required to solve for x and y, given data from reaction of J with sodium metal and combustion analysis.
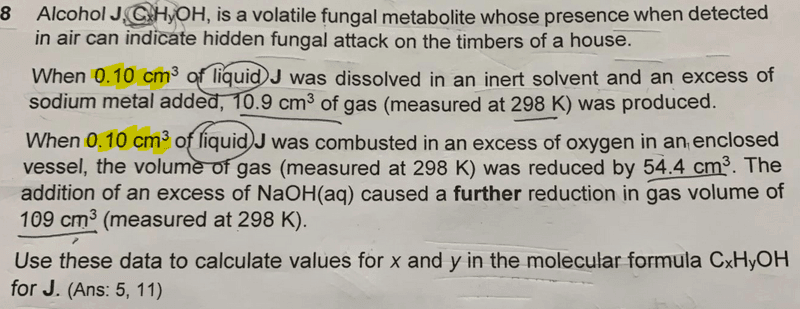
Let's first break this question down into diagram form:
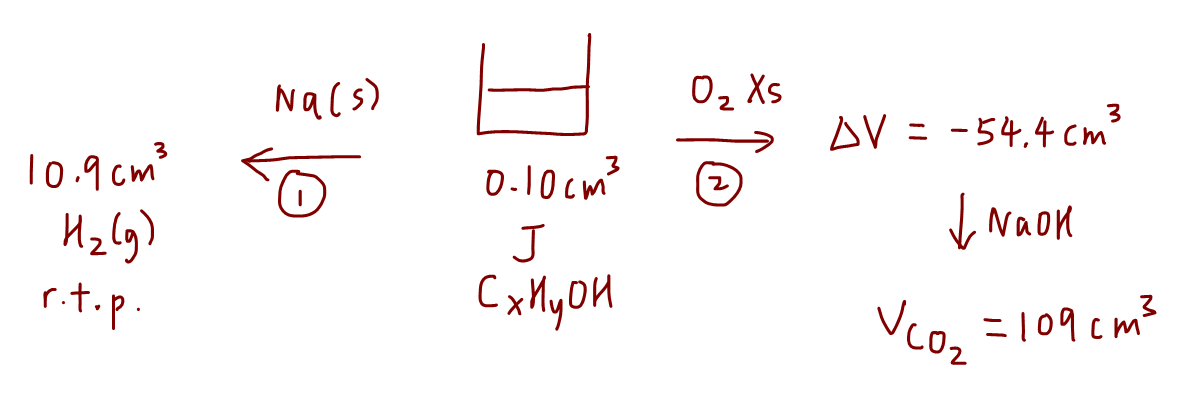
The first reaction with sodium will give H2 gas. So from the volume of H2 evolved we can determine the number of moles of alcohol J.
The second combustion reaction of the same volume (and hence same amount) of J with oxygen causes a decrease in volume by 54.4cm3, and a further reduction of 109cm3 when NaOH is added.
The reduction of volume with NaOH is due to acidic CO2 that is absorbed, so we already know that volume of CO2 is 109cm3.
1. Reaction with sodium
We need to write down the balanced equation between alcohol J and sodium to form salt and hydrogen gas.
We can then determine the amount of H2 produced and compare mole ratio to find amount of J.
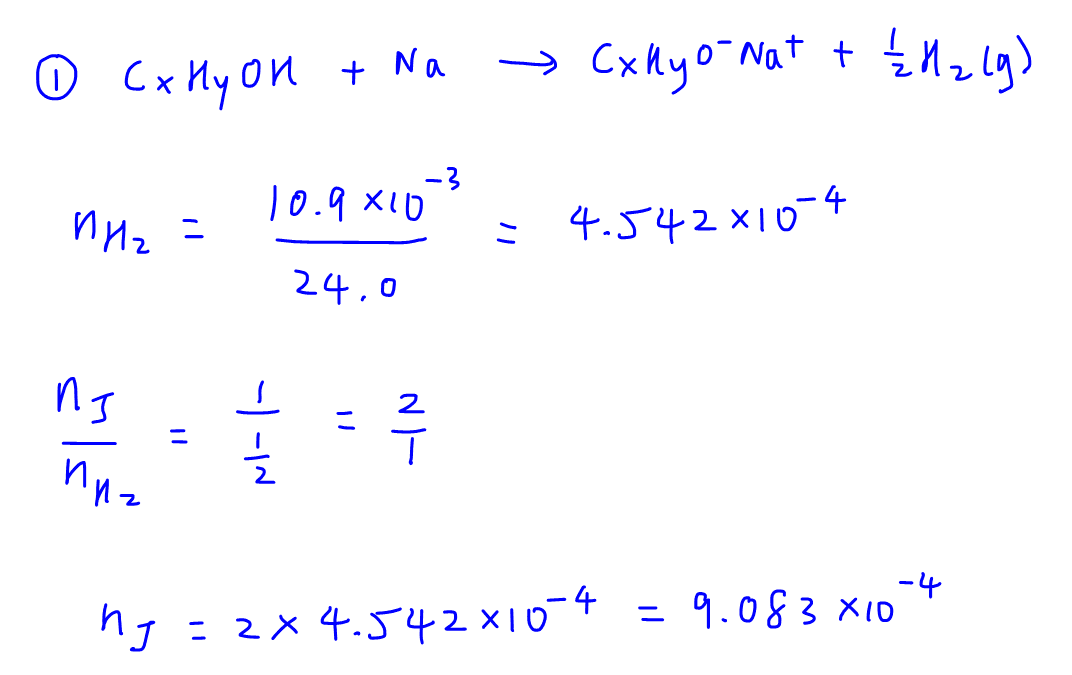
2. Combustion Analysis
We need to balance the equation involving combustion of alcohol J (CxHyOH) to form CO2 and H2O, which is a bit tedious and different from normal combustion analysis questions involving hydrocarbons (CxHy).
Since we already know the volume of CO2 produced is 109cm3, we can work out the volume of oxygen reacted from change in volume.
We can then convert the volumes of CO2 and O2 reacted to number of moles.
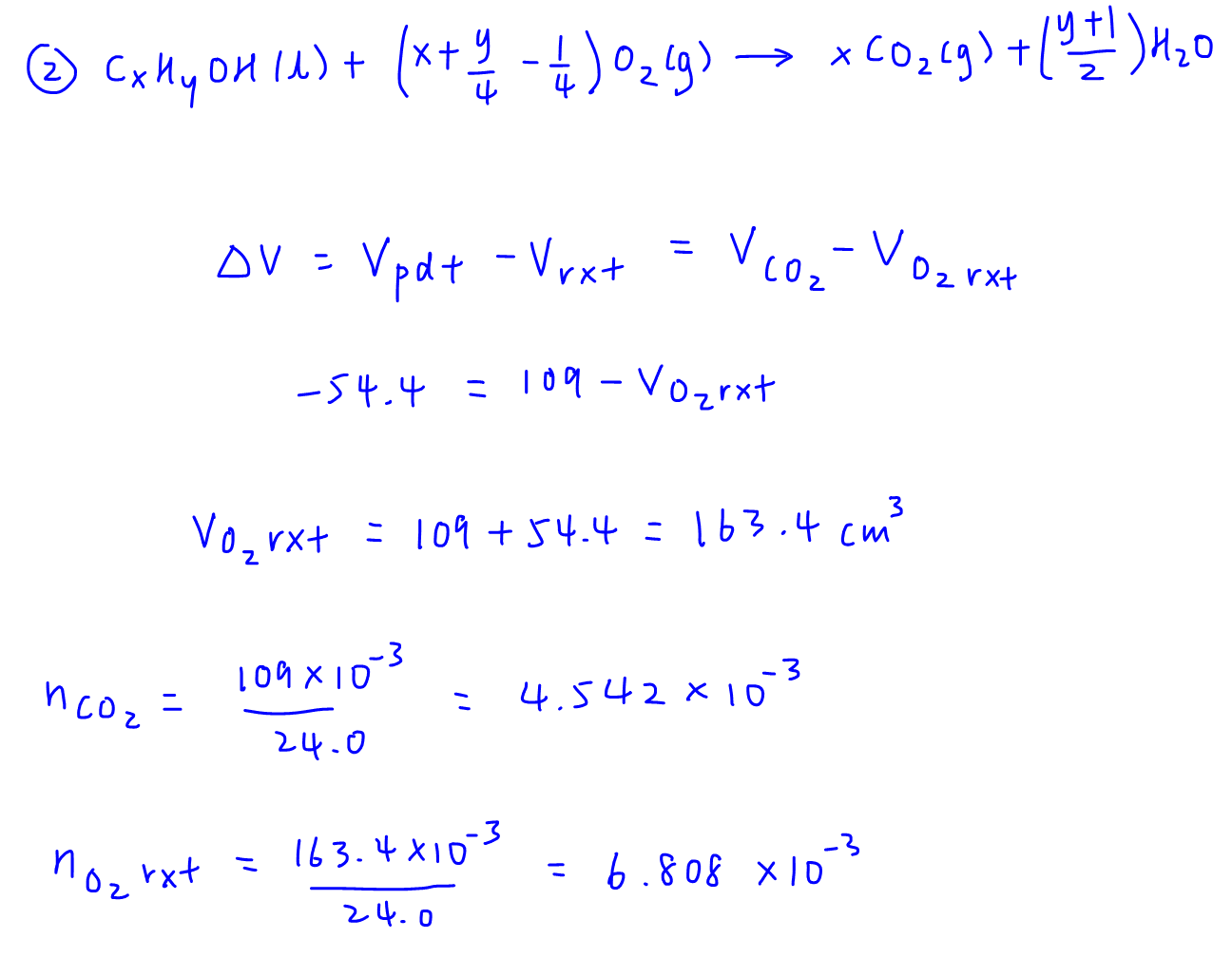
Take note for this question we cannot compare volume ratio as alcohol J is a liquid and comparing volume ratio only works on gases (Avogadro's Law).
3. Comparing Mole Ratio
We can now combine information from both experiments and write out 2 mole ratios for the balanced equation in terms of:
a. coefficients x and y
b. number of moles determined from both experiments
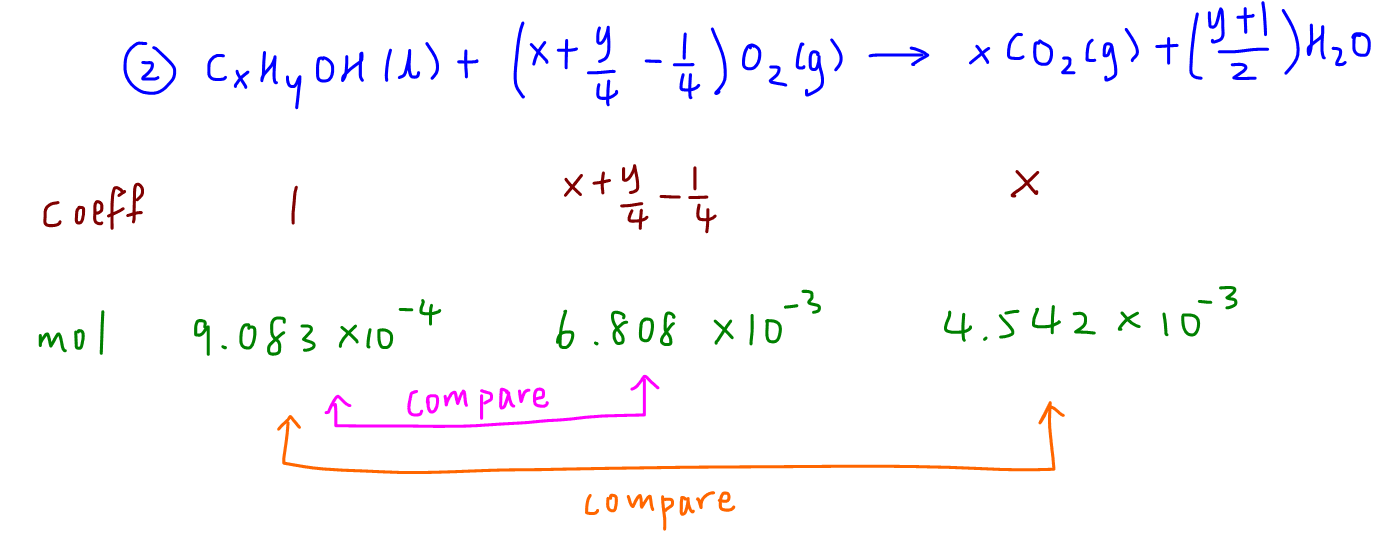
To determine x, we compare mole ratio of CO2 to alcohol J.
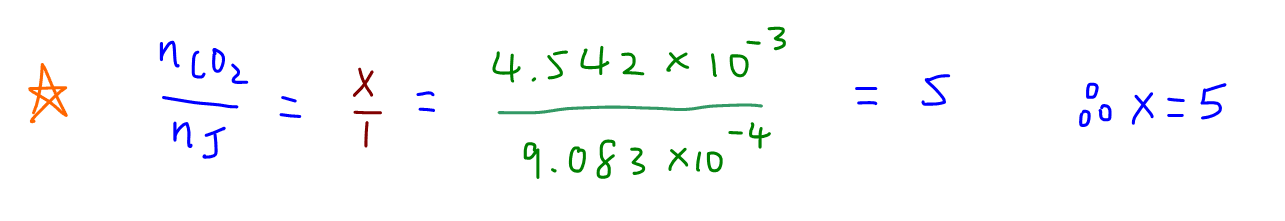
To determine y, we compare mole ratio of O2 reacted to alcohol J.
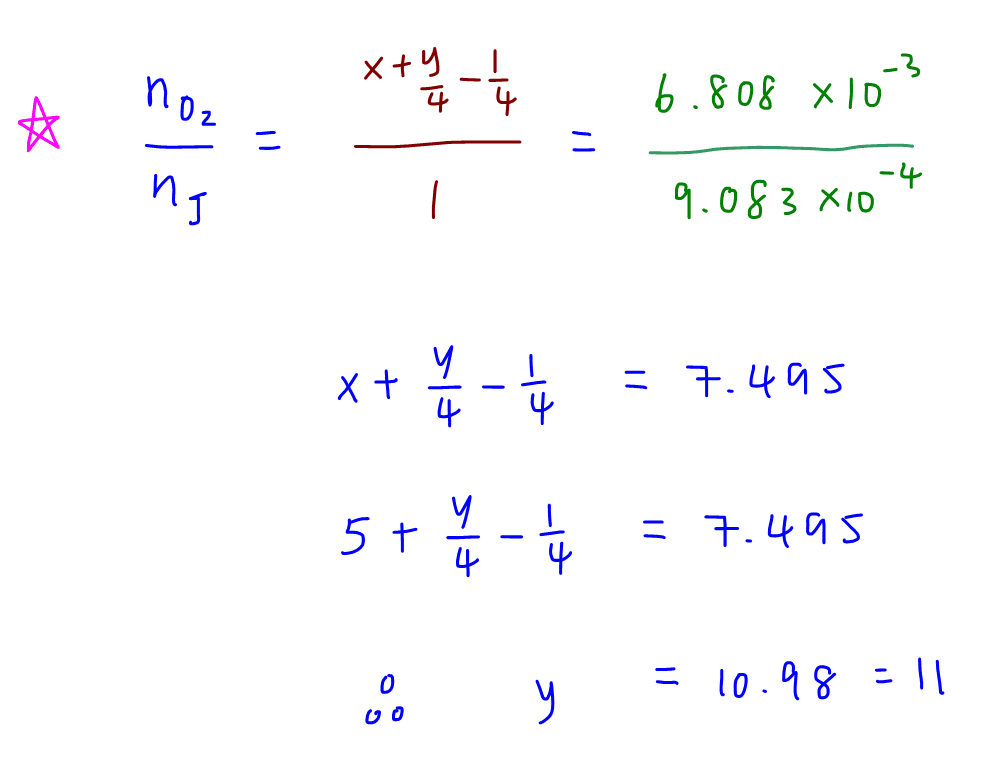
So we can determine x = 5, y = 11 and the molecular formula for alcohol J is C5H11OH.
For the detailed step-by-step discussion on how to determine molecular formula for alcohol J, check out this video!
Topic: Mole Concept, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's reputable A Level Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online chemistry classes!
