2019 A Level H2 Chemistry Paper 1 Question 11 - Determine Mole Ratio of Pb to B in Solder Glass
Let Chemistry Guru, Singapore's renowned JC Chemistry tuition centre, guide you through 2019 A Level H2 Chemistry Paper 1 Question 11.
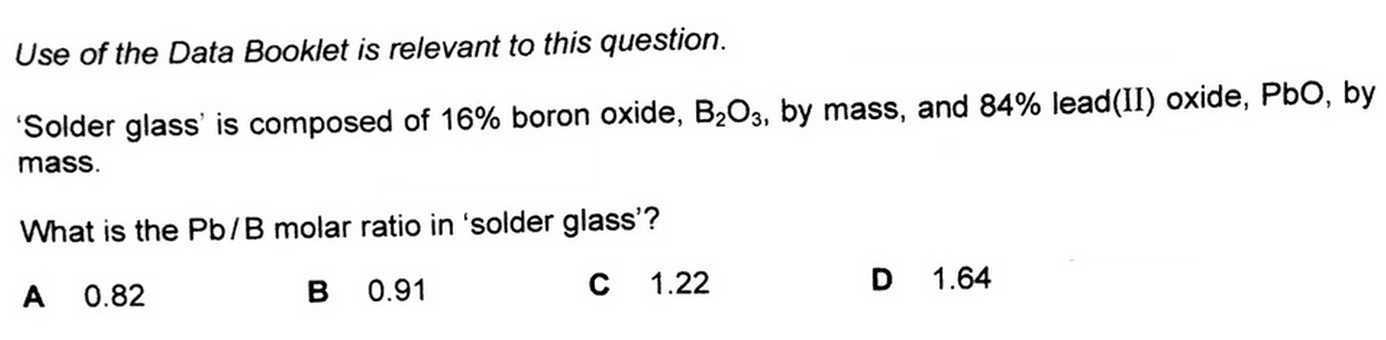
We are given that solder glass contains 16% of B2O3 and 84% of PbO.
Hence in 100g, there will be 16g of B2O3 and 84g of PbO.

From mass of B2O3, we can find its number of moles by dividing by molar mass.
We then compare mole ratio between B to B2O3 and determine number of moles of B.

Similarly we can determine the number of moles of Pb from 84g of PbO.
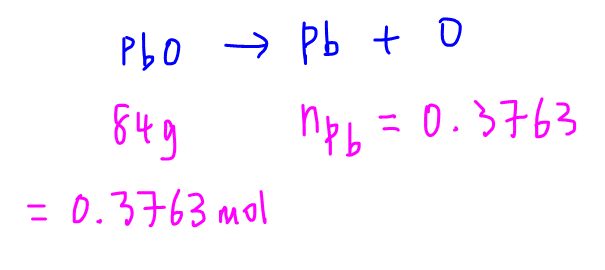
Finally we can calculate the mole ratio of Pb to B.

Hence the answer to this question will be option A.
Topic: Mole Concept, Physical Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2019 A Level H2 Chemistry Paper 1
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online tuition classes!
