Nucleophilic Addition, Carbonyl Compound Mechanism - Organic Chem
In this video we want to describe the nucleophilic addition mechanism of carbonyl compounds, aldehydes and ketones.
Let Chemistry Guru, Singapore's top JC Chemistry tuition centre, guide you through an example.
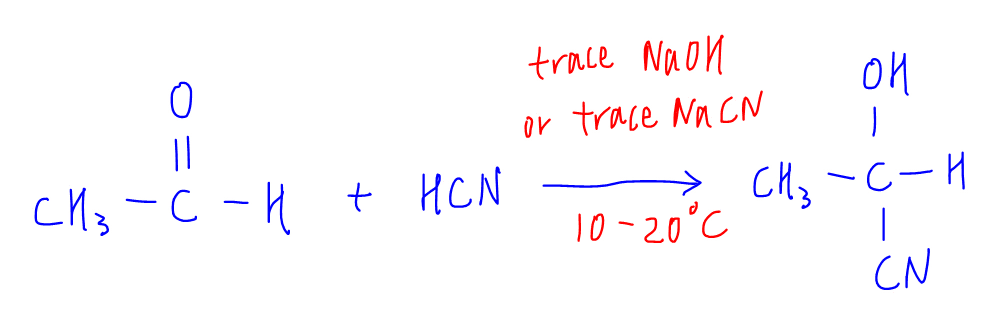
This reaction is done using trace NaOH or trace NaCN at 10 to 20 degree celsius.
1. Generation of Nucleophile
The first step is to generate the nucleophile.
If trace NaOH is used, there will be an acid-base reaction between NaOH and HCN to generate the nucleophile CN-.
If trace NaCN is used, it will be a simple dissociation to generate CN-.
The first step is the fast step.

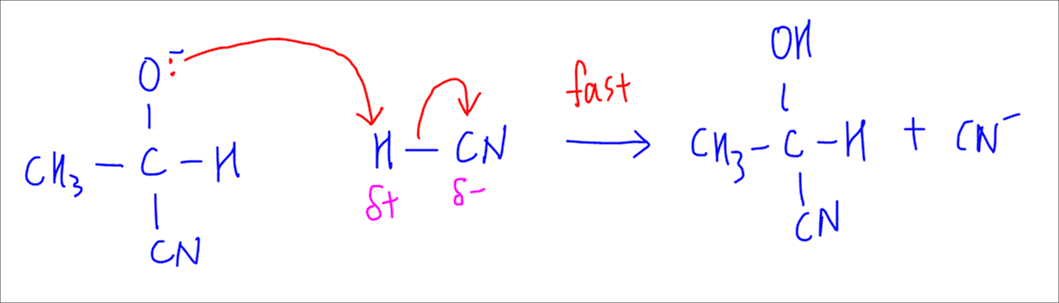
2. Nucleophilic Attack
In the second step the nucleophile will attack carbonyl carbon to form a carbon-carbon bond.
The pi bond between carbon and oxygen will break and both electrons will be transferred to oxygen, forming an alkyoxide.
This second step is the slow step.

3. Protonation and Regeneration of Catalyst
The alkyoxide oxygen will attack the reactant HCN and extract a proton or H+ from it, forming the product cyanohydrin.
CN- is regenerated in this process.
This final step occurs readily and is the fast step.
For the detailed step-by-step discussion on how to draw this mechanism, check out this video!
Topic: Carbonyl Compounds, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's leading JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online chemistry classes!
