Nucleophilic Substitution of Amine
Here's the question for discussion in this video created by Chemistry Guru, Singapore's leading JC Chemistry tuition centre.
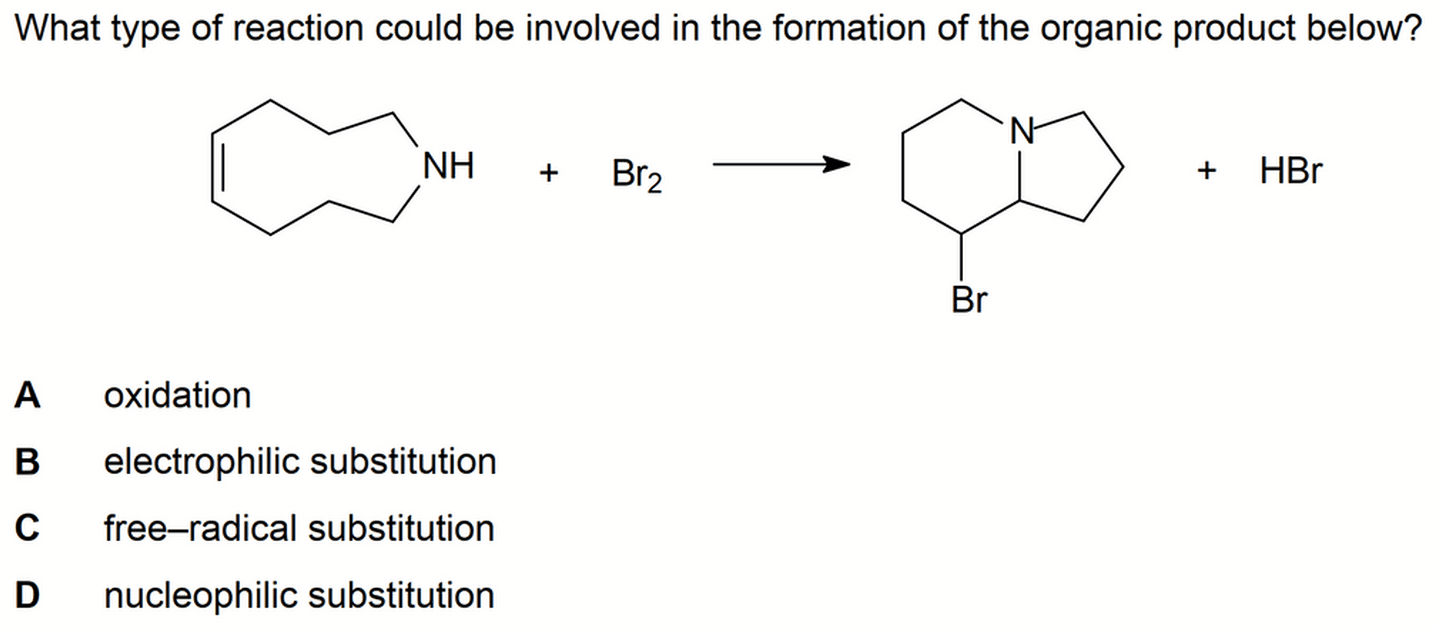
The reactant has 2 functional groups - alkene and secondary amine.
The product has a bromoalkane and tertiary amine.
Interestingly the big cyclocompound in the reactant has been converted to 2 smaller cyclocompounds in the product.
Let's try to break down and figure out the reactions in this question.
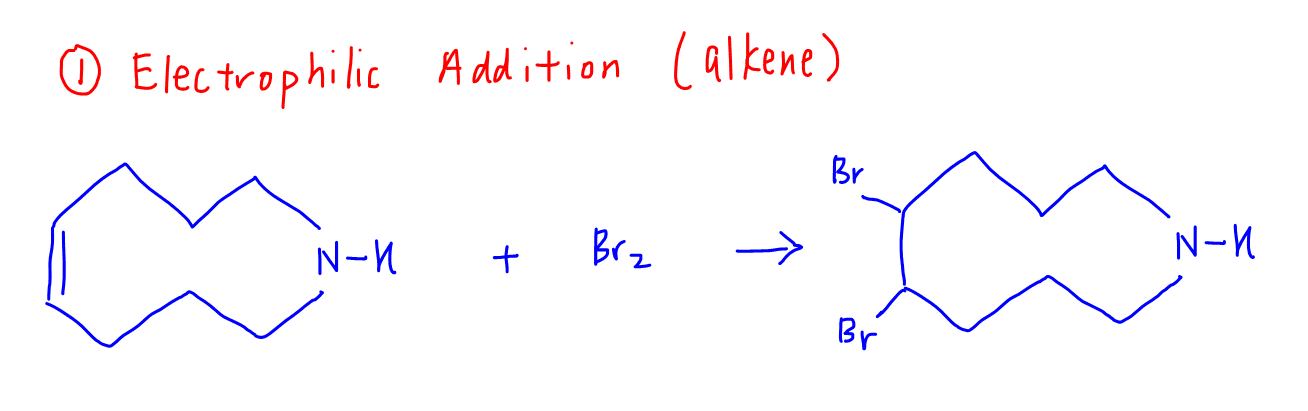
1. Electrophilic Addition of Alkene
The first step is pretty straightforward - electrophilic addition of alkene with bromine to form dibromoalkane.
2. Nucleophilic Substitution of Amine
The second step is the nucleophilic substitution of secondary amine to form tertiary amine.
Therefore the answer to this question will be D (nucleophilic substitution).
But the interesting part is to figure out how the big cyclocompound is converted to the 2 smaller ones.
Let's draw the mechanism in detail.
2a. Nucleophilic Attack on bromoalkane and formation of ammonium intermediate
The amine nitrogen uses its lone pair and acts as a nucleophile to attack one of the bromoalkane carbons.
It doesn't really matter which bromoalkane is attacked, since the reactant is symmetrical.
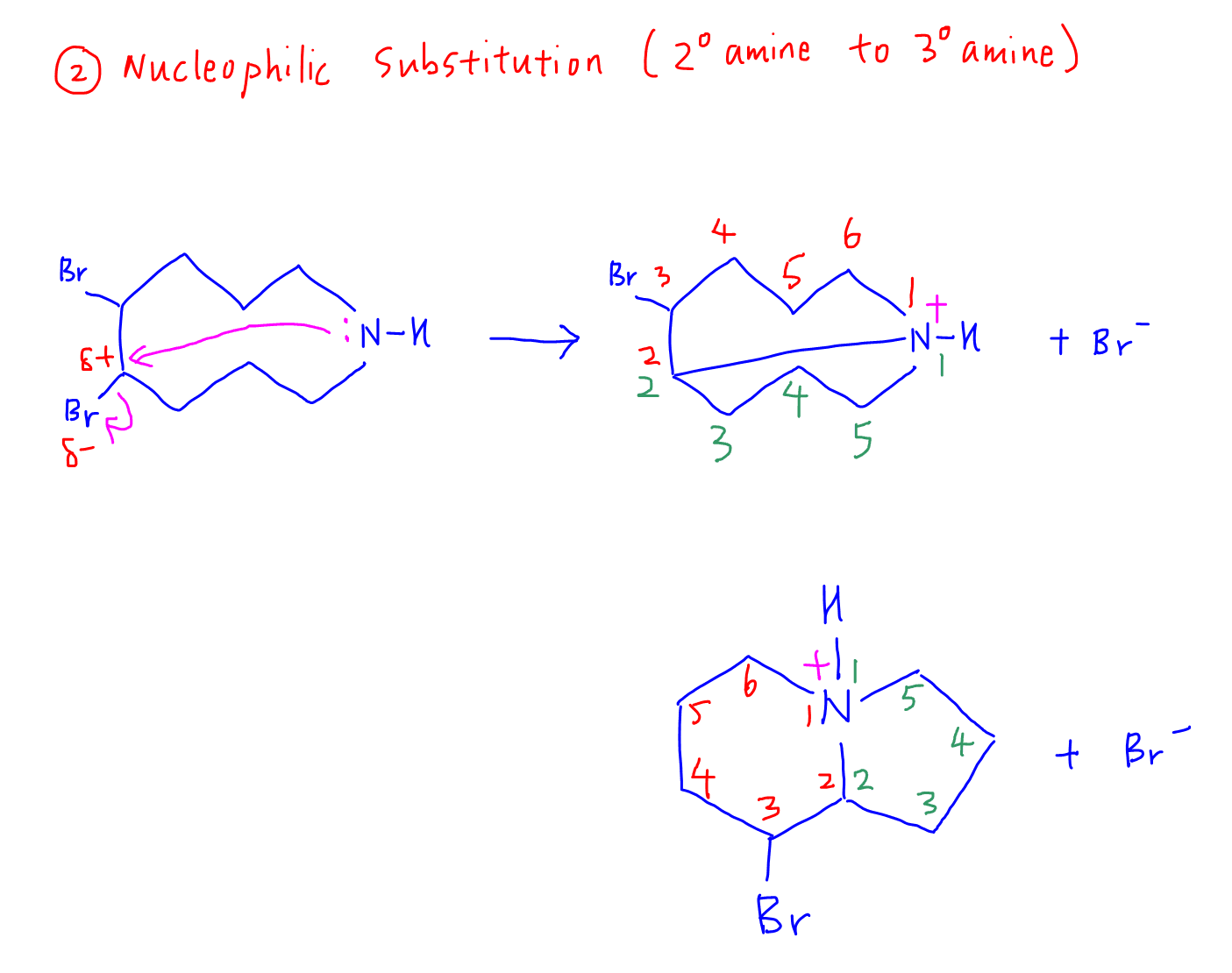
When the C-N bond is formed, we need to visualise what the new product will look like with the 2 smaller cyclocompounds.
It is very helpful to number the members in each cyclocompound to figure out how many members are there in each cyclostructure.
In this case we have a 5-member (pentagon) and 6-member (hexagon) cyclocompound.
The intermediate formed is an ammonium salt and bromide ion.
2b. Deprotonation and formation of products
The bromide ion will attack the ammonium hydrogen, which deprotonates to form the tertiary amine and HBr products.

Topic: Nitrogen Compounds, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online tuition classes!
