Optical Activity of Nucleophilic Addition Product
Let's consider the nucleophilic addition reaction of carbonyl compounds.
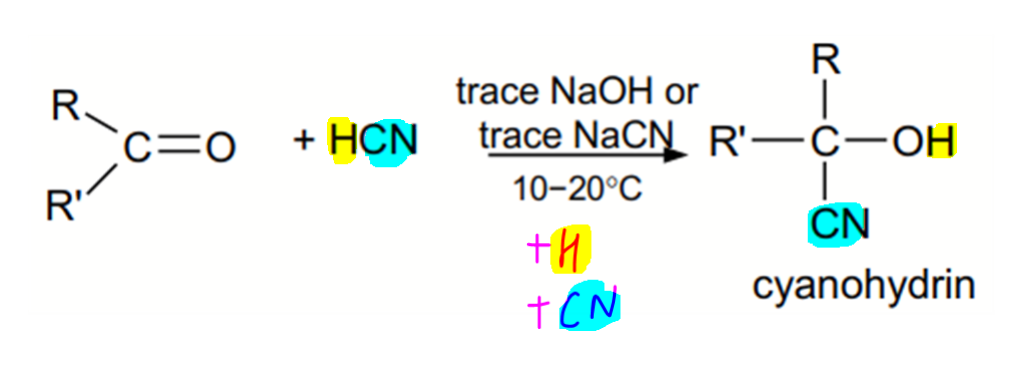
Notice the cyanohydrin product has a chiral carbon which is bonded to 4 different groups.
Does this mean the product is optically active?
Check out this video lesson on optical isomerism and cis-trans isomerism.
Interestingly the product is optically inactive due to a racemic mixture being formed.
We need to use the mechanism to understand why this happens.
Here's the detailed explanation of nucleophilic addition mechanism of carbonyl compounds.
Since the carbonyl carbon is trigonal planar, both sides of the sp2 carbon are equally exposed.
Hence the nucleophile CN- can attack the carbonyl carbon from both sides to equal extent to form an equimolar mixture of optical isomers, ie racemic mixture which is optically inactive.
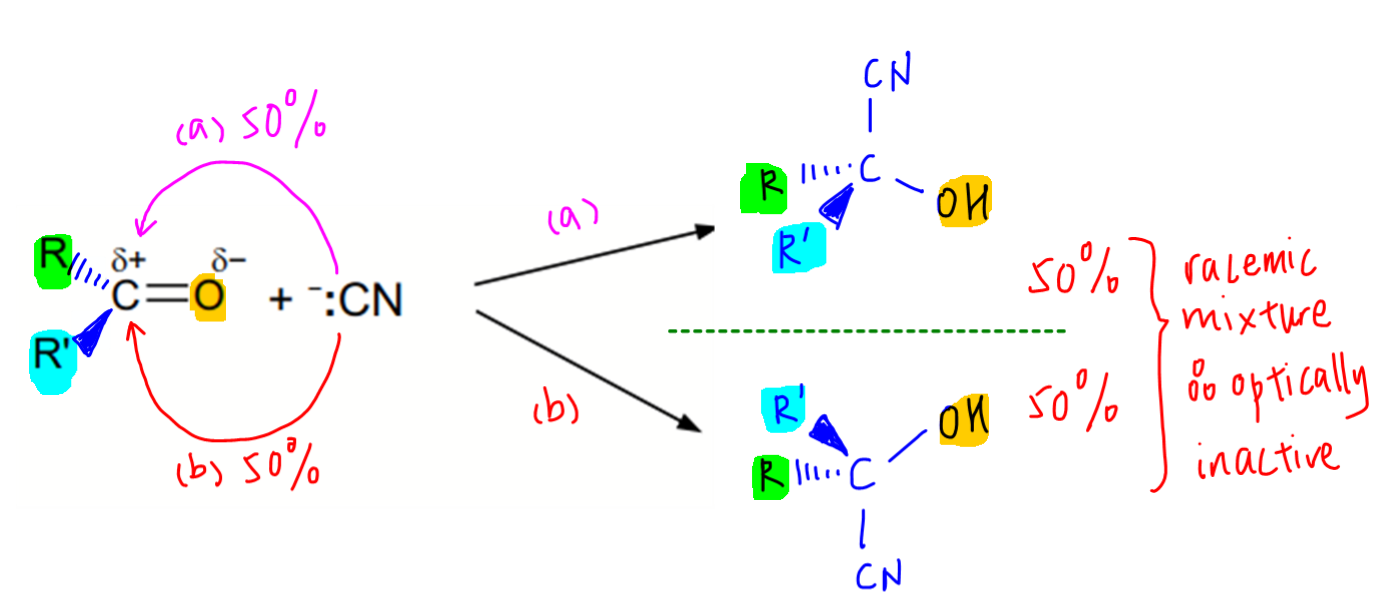
This outcome is also seen in SN1 mechanism of halogenoalkanes where the carbocation formed is trigonal planar.
Learn all about nucleophilic substitution mechanism of halogenoalkanes.
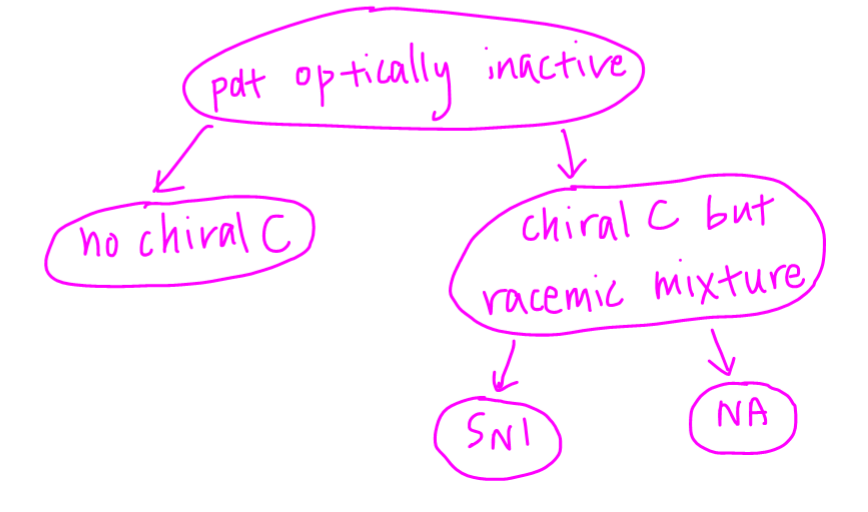
So when we are given that the product is optically inactive, the possible reasons are:
1. No chiral carbon
2. Presence of chiral carbon but racemic mixture due to sp2 hybridised carbon being attacked eg SN1 and NA
Topic: Carbonyl Compounds, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
