Organic Synthesis Via Step Up Reaction
Organic synthesis is a very common type of question in A Level Organic Chemistry, where students need to deduce a synthesis route to convert compound A to compound B in a maximum of 4 reaction steps.
Very often we will encounter an increase in the number of carbon in the product, so we need to use a step up reaction to introduce a carbon during the synthesis.
Let's have an example.
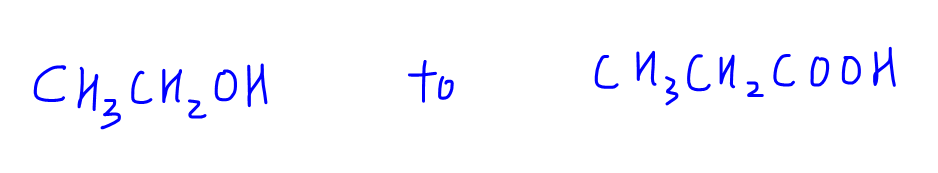
We notice there is an increase in carbon from 2 carbons in ethanol to 3 carbons in propanoic acid.
So how should we go about converting ethanol to propanoic acid? Let's go through some important points before we tackle this question.
1. Step Up Reaction via Nitrile
First we have to know the only way in syllabus to add a carbon to an organic compound is via a nitrile (CN).
Next we need to remember there are TWO ways to introduce the nitrile:
a. Nucleophilic Substitution of Halogenoalkane, where a halogen is REPLACED with a nitrile
b. Nucleophilic Addition of Carbonyl Compound, where a nitrile is ADDED to the carbonyl carbon and the carbonyl carbon becomes an alcohol.
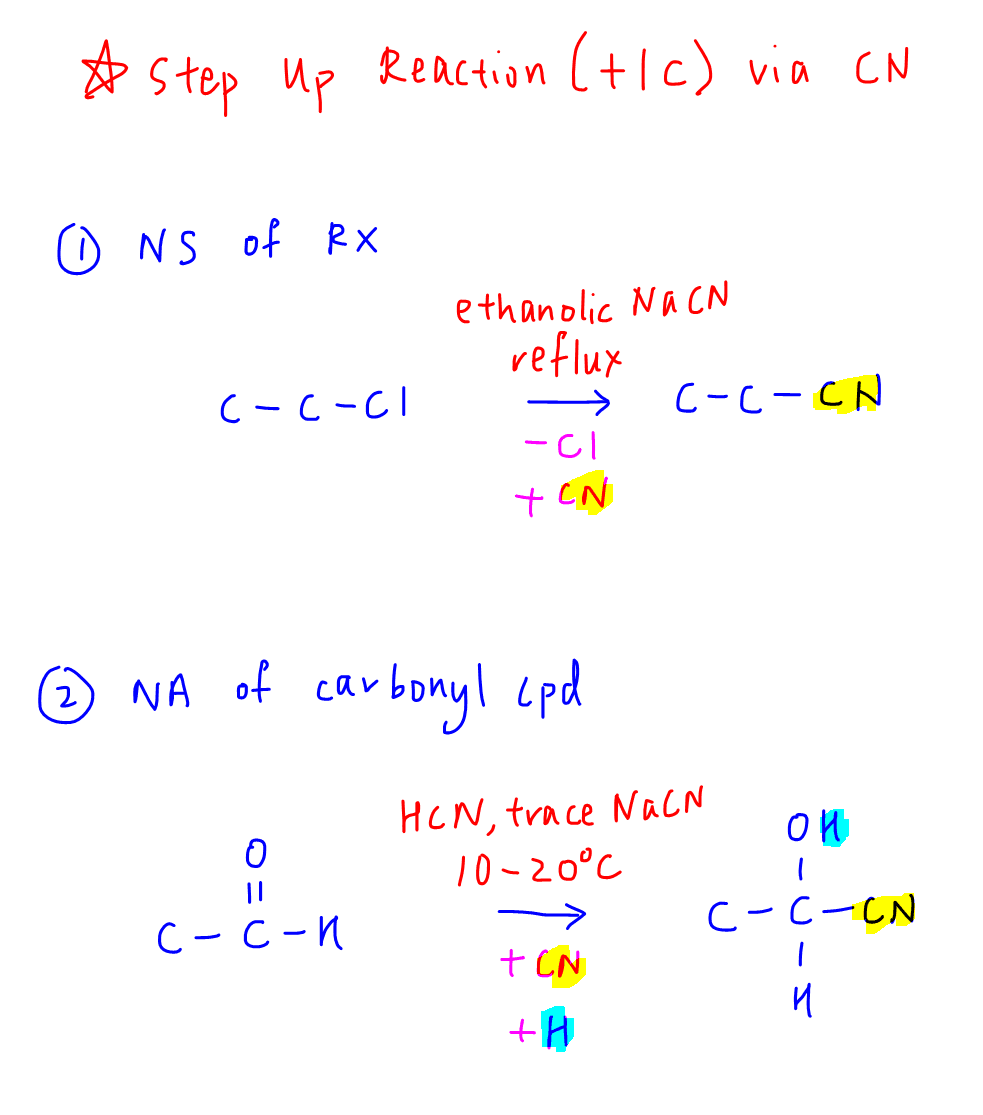
Based on the number of functional groups in the product, we need to figure out whether we should do a step up via nucleophilic substitution or nucleophilic addition.
These 2 reactions are not interchangeable as the number of functional groups in the products are different.
2. Reactions of Nitrile
Usually questions will not leave the nitrile as it is (this would be too obvious!), so we need to know what functional groups nitrile can be converted to.
a. Primary amine via reduction
b. Carboxylic acids via hydrolysis
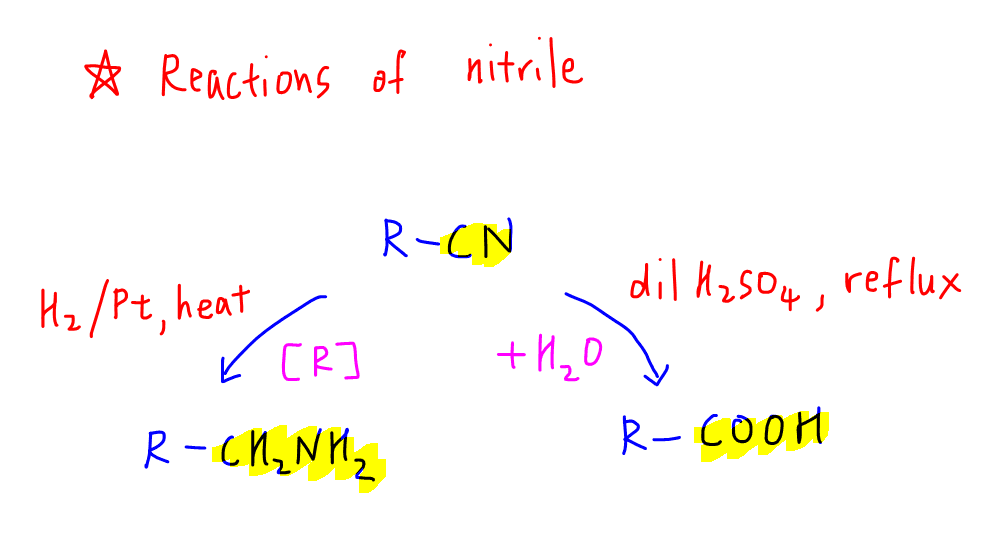
Hydrolysing nitrile to acid is much more popular as carboxylic acids can be converted into many other functional groups in Organic Chemistry hence is more useful for Organic synthesis.
3. Retrosynthesis
Finally we can look at the question and plan backwards, a technique known as retrosynthesis.
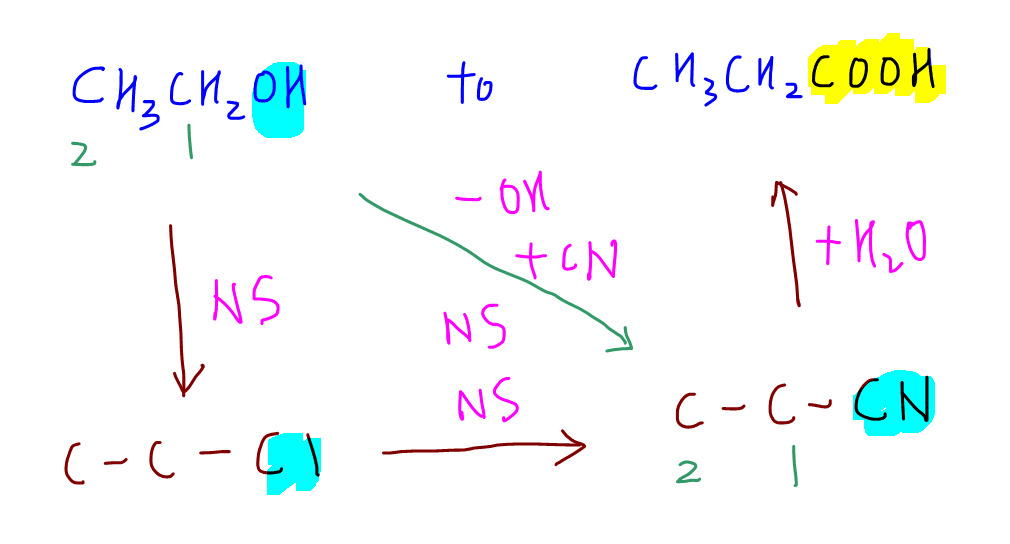
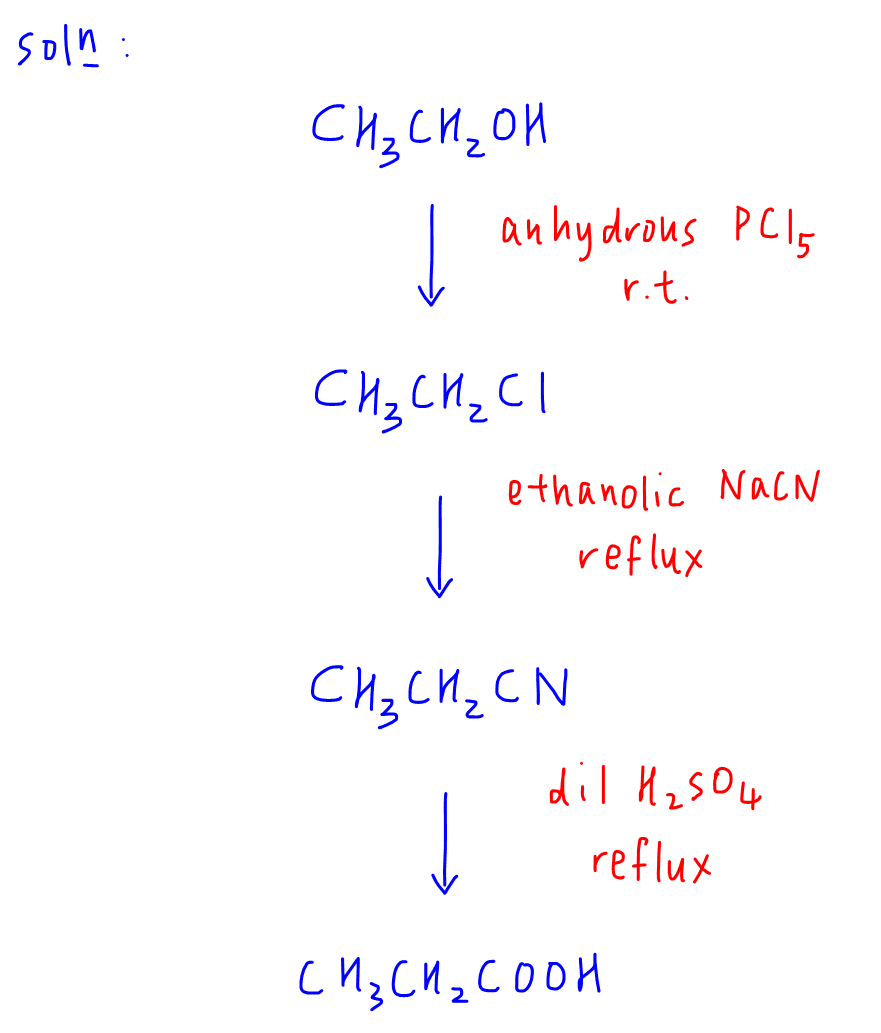
a. From the product propanoic acid, the acid functional group is the closest to nitrile so we can work backwards and deduce that propanoic acid is formed from propane nitrile via hydrolysis.
b. Comparing propane nitrile with starting compound ethanol, we can deduce we need to REPLACE -OH group with -CN group, hence we need to do a step up reaction via nucleophilic substitution of halogenoalkane, chloroethane.
c. Finally we can get chloroethane from ethanol via another nucleophilic substitution reaction.
4. Solution
Once the planning is done we can work out the synthesis route and write down the reagents and conditions for each reaction.
For the detailed step-by-step discussion on how to answer Organic synthesis questions via step up reaction, check out this video!
Topic: Halogenoalkanes, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's reputable A Level Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online tuition classes!
