2019 A Level H2 Chemistry Paper 1 Question 22 - Oxidation of Alcohols for Compound Q
Here's 2019 A Level H2 Chemistry Paper 1 Question 22.

We need to deduce the product formed when compound Q is heated under reflux with an excess of acidified potassium dichromate (VI).
Notice there are only alcohol functional groups in compound Q.
Different types of alcohols are oxidised to different extent when reacting with acidified dichromate, heated under reflux.
Primary alcohols are oxidised to carboxylic acids.
Note that primary alcohols can be oxidised to aldehydes but only with acidified dichromate, heating under reflux with immediate distillation.
Secondary alcohols are oxidised to ketones.
Tertiary alcohols cannot be oxidised.

Check out my previous video lesson for the detailed discussion of oxidation of different types of alcohols.
So we can now run through the options and figure out our answer!
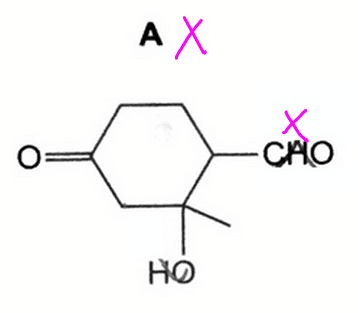
Option A is wrong as the primary alcohol is not oxidised to aldehyde.
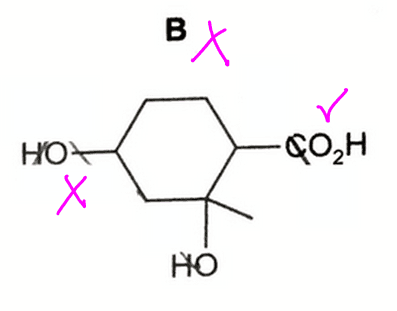
Option B is wrong as the secondary alcohol should be oxidised.
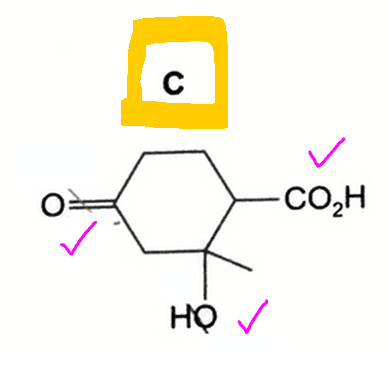
Option C is the correct answer.
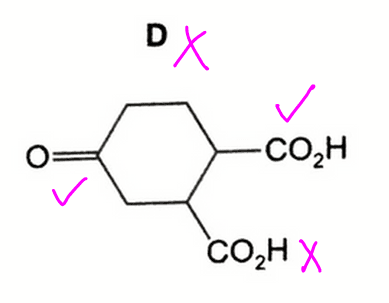
Option D is wrong as the tertiary alcohol should not be oxidised.
Hence the answer to this question is option C.
Topic: Alcohols, Organic Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2019 A Level H2 Chemistry Paper 1
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online tuition classes!
