Oxidation of Alkyl Benzene
In this video created by Chemistry Guru, Singapore's leading JC Chemistry tuition centre, we want to discuss the oxidation of alkyl benzene using KMnO4 in dilute H2SO4, heat under reflux.
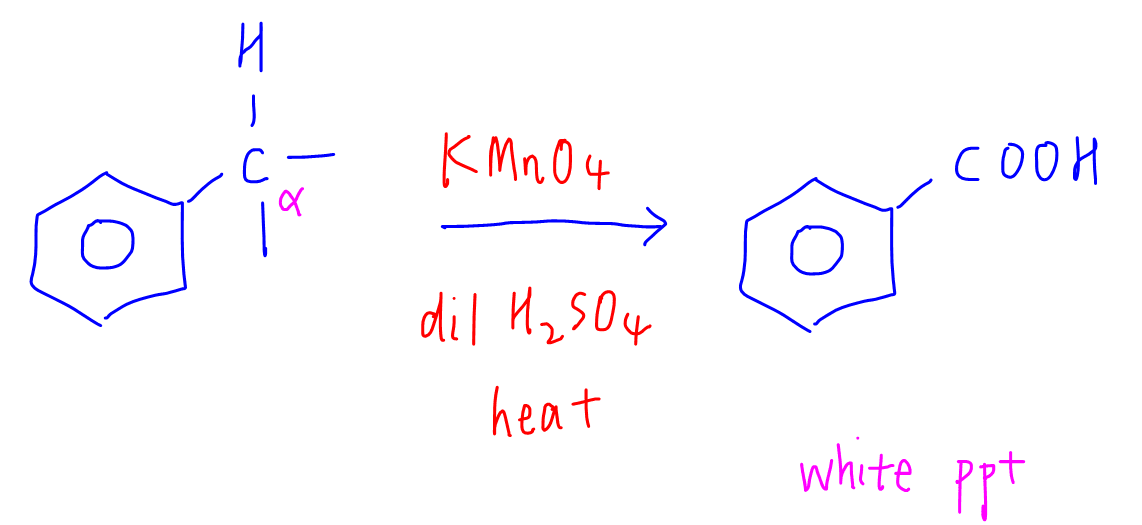
The criteria for alkylbenzene to be oxidised to benzoic acid is the alpha carbon (carbon directly bonded to benzene) must have minimum 1 H.
The observation for this reaction will be the decolourisation of purple KMnO4 and formation of white precipitate benzoic acid.
In A Level Chemistry Syllabus, the rest of the groups attached to alpha carbon are usually discarded and we do not need to determine what they are oxidised to, unless it is one carbon then it'll be oxidised to CO2.
Let Chemistry Guru, Singapore's top JC Chemistry tuition centre, guide you through a few examples:
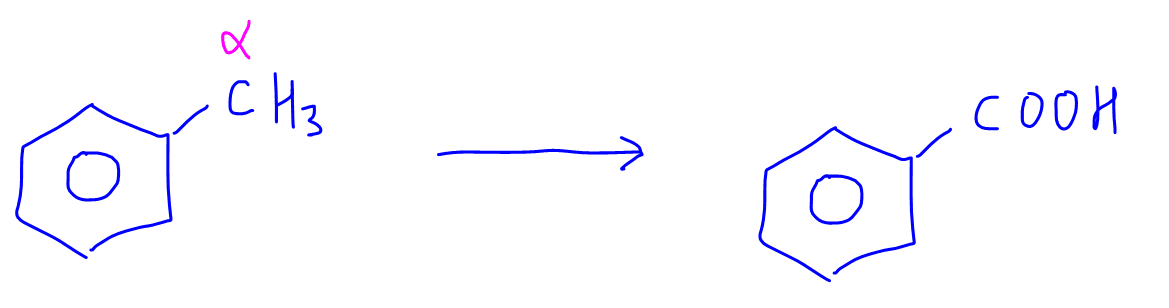


Notice for the last example, there is no oxidation since the alpha carbon has no hydrogen attached to it.
Quiz: Oxidation using Different Oxidising Agents
Take a look at this compound.
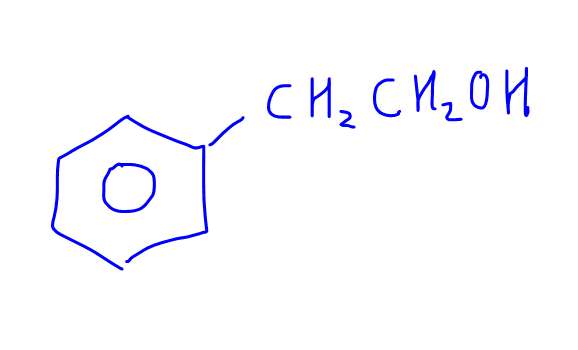
If the above compound is subjected to the following oxidising agents, would they form the same product?
either K2Cr2O7, dilute H2SO4, heat
or KMnO4, dilute H2SO4, heat
Test yourself first and check out this video to find out if you got this correct!
Topic: Benzene, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's reputable A Level Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online chemistry classes!
