Determining Oxidation State of Carbon in Organic Compounds
Sometimes in Organic Chemistry we are required to determine oxidation state of carbon of a particular functional group.
Let's discuss how to do this.
First we need to be familiar with the definition of oxidation state and how it is determined.
I have a previous video lesson where I discussed how to determine oxidation state or oxidation number, do check it out if you are not sure!
To determine the oxidation state of carbon in an organic compound, we need to break all the bonds around carbon and decide what charge carbon would acquire if it were an ion.
The following table is useful to assign the charges.
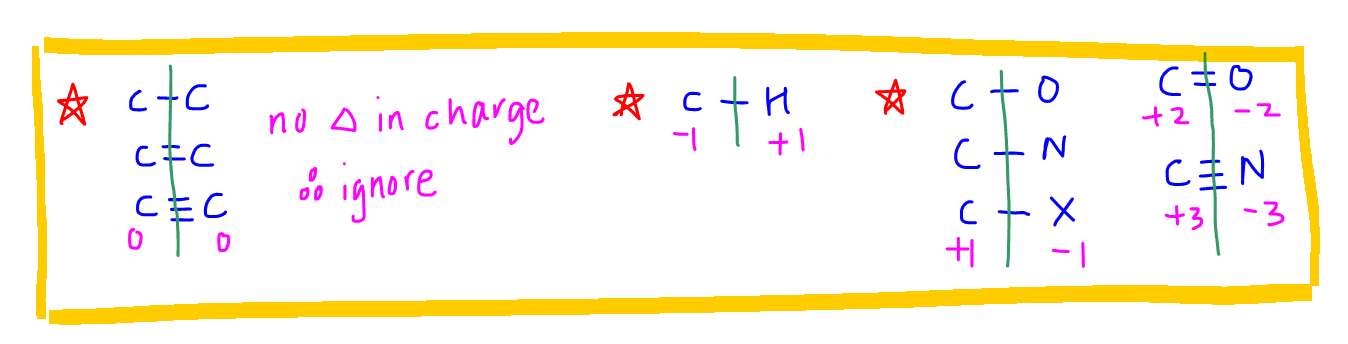
Notes:
1. We can ignore carbon-carbon bonds as there is no difference in electronegativity and breaking of any carbon-carbon bond will not result in a charge for carbon.
2. Carbon will acquire a negative charge when C-H bond is broken as C is more electronegative than H.
3. Carbon will acquire a positive charge when C-O, C-N or C-X bond is broken as C is less electronegative than most of the other elements in organic compounds.
Let's have a few examples to illustrate this.
1. CH3CH2CH3

Drawing the displayed structure will help us visualise the breaking of the bonds better.
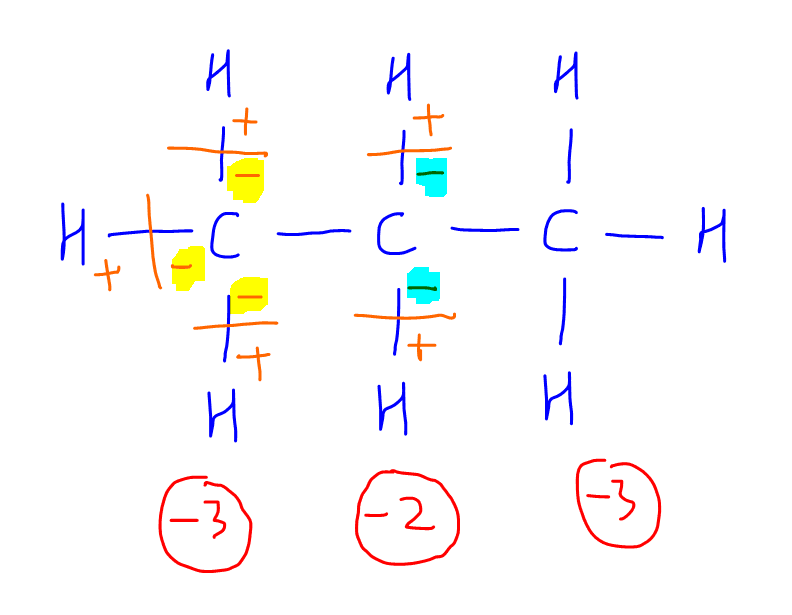
From the diagram we can see that the first carbon will acquire a -3 charge when all its bonds are broken, so its oxidation state will be -3.
The second carbon will acquire a -2 charge when all its bonds are broken, so its oxidation state will be -2.
2. CH3CH(OH)CH2OH
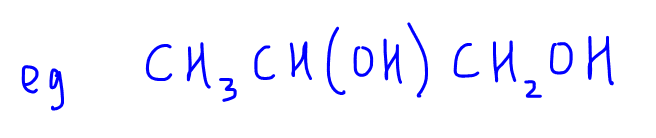
We can determine that the oxidation state of the secondary alcohol carbon is zero, and that of primary alcohol carbon is -1.
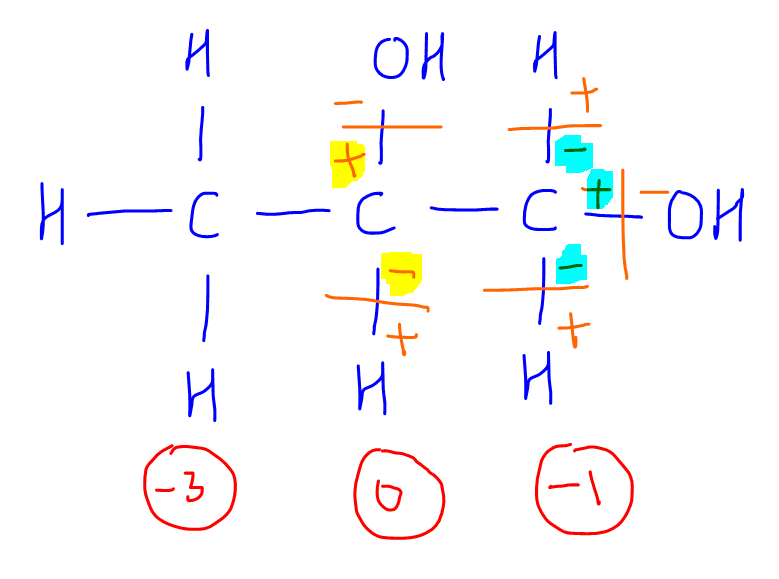
3. CH3COCOOH

We can determine that the oxidation state of ketone carbon is +2 and that of carboxylic acid carbon is +3.
For the detailed step-by-step discussion on how to determine oxidation state of carbon in organic compounds, check out this video!
Topic: Introduction to Organic Chemistry, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's reputable A Level Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online chemistry classes!
