Oxidation of Primary Alcohol to Aldehyde
In this JC2 webinar we want to discuss the oxidation of primary alcohol to aldehyde.
Let Chemistry Guru, Singapore's renowned JC Chemistry tuition centre, guide you through the reagents and conditions.
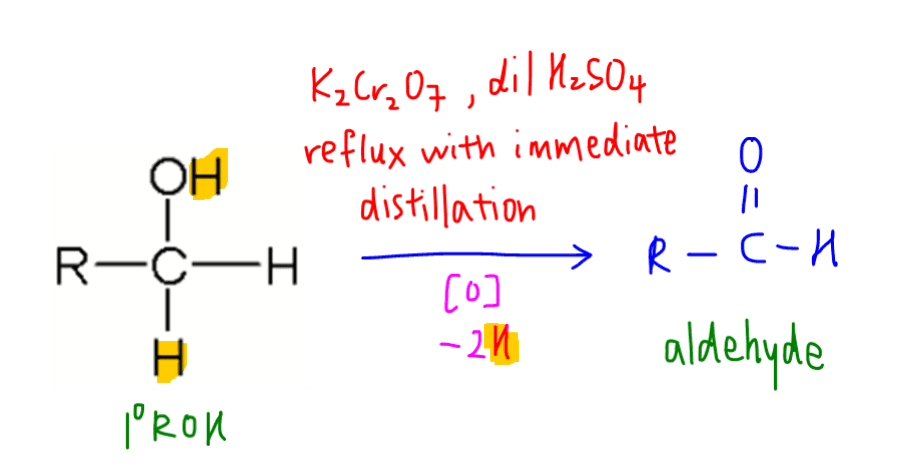
Note that the alcohol carbon and oxygen will lose 1 hydrogen each.
This is consistent with the definition of oxidation via loss of hydrogen.
The product aldehyde is not stable to oxidation and can be further oxidised to carboxylic acid.
Hence we need to control the oxidation in order to get aldehyde.
We cannot use potassium manganate (VII) or KMnO4 in this reaction.
KMnO4 is too strong and will oxidise primary alcohol to acid directly.
Immediate distillation is also required, or the product will also be carboxylic acid.
Since we can use reflux with immediate distillation to remove aldehyde from this reaction mixture, does it mean we can always remove the product from any reaction via distillation?
To answer this question we need to understand the basis of separation.
Separation of mixture is usually based on the difference in physical properties of the components in the mixture.
Examples:
1. If mixture is solid and liquid with different physical states, we can separate them via filtration.
2. If mixture is 2 miscible liquids with different boiling points, we can separate them via distillation.
3. If mixture has different solubility in organic solvent and water, we can separate them via extraction via organic solvent or water.
For distillation, the liquid with the lower boiling point will be distilled out first.
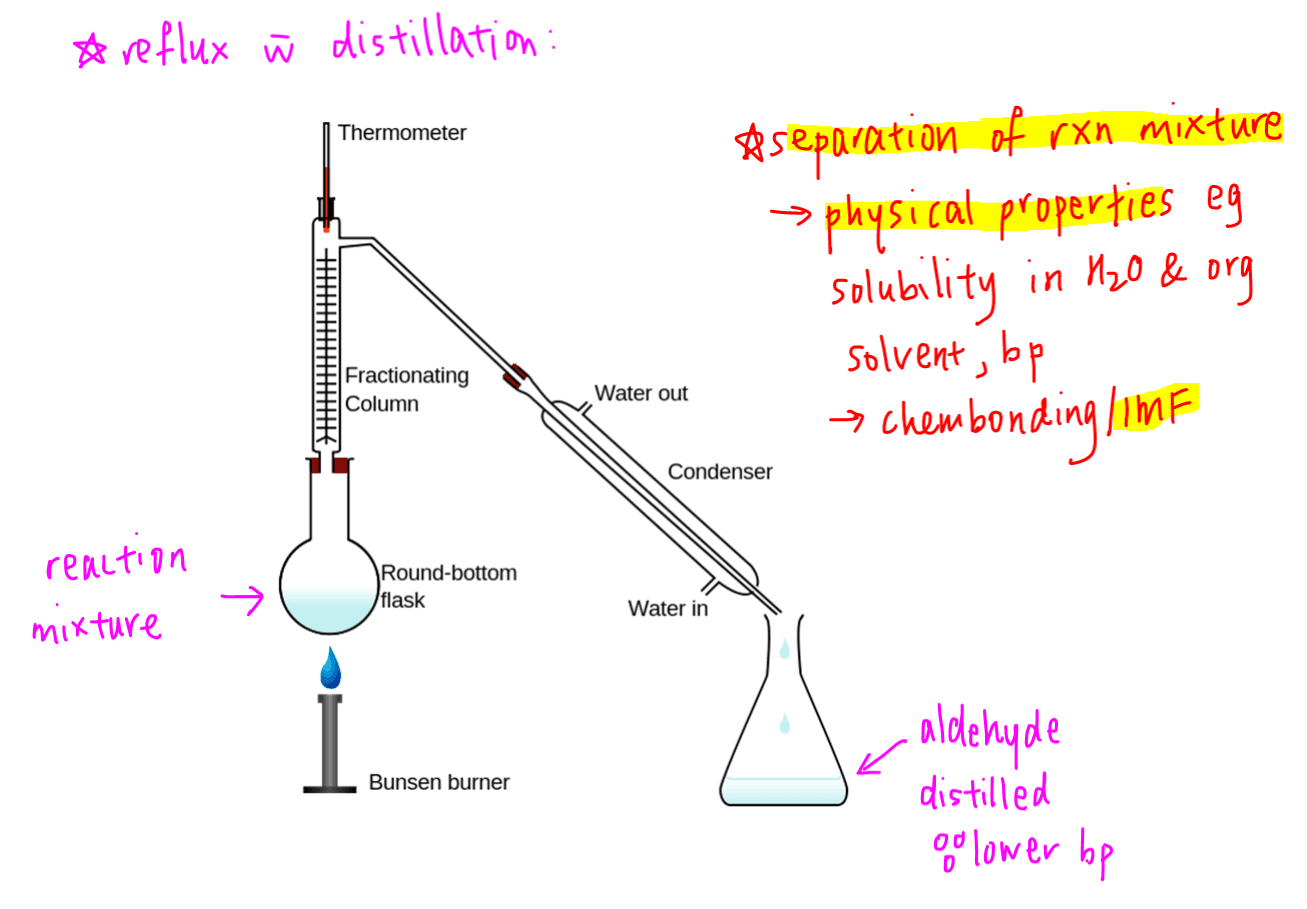
So for the oxidation of primary alcohol to aldehyde, the aldehyde will be distilled first due to its lower boiling point as compared to alcohol.
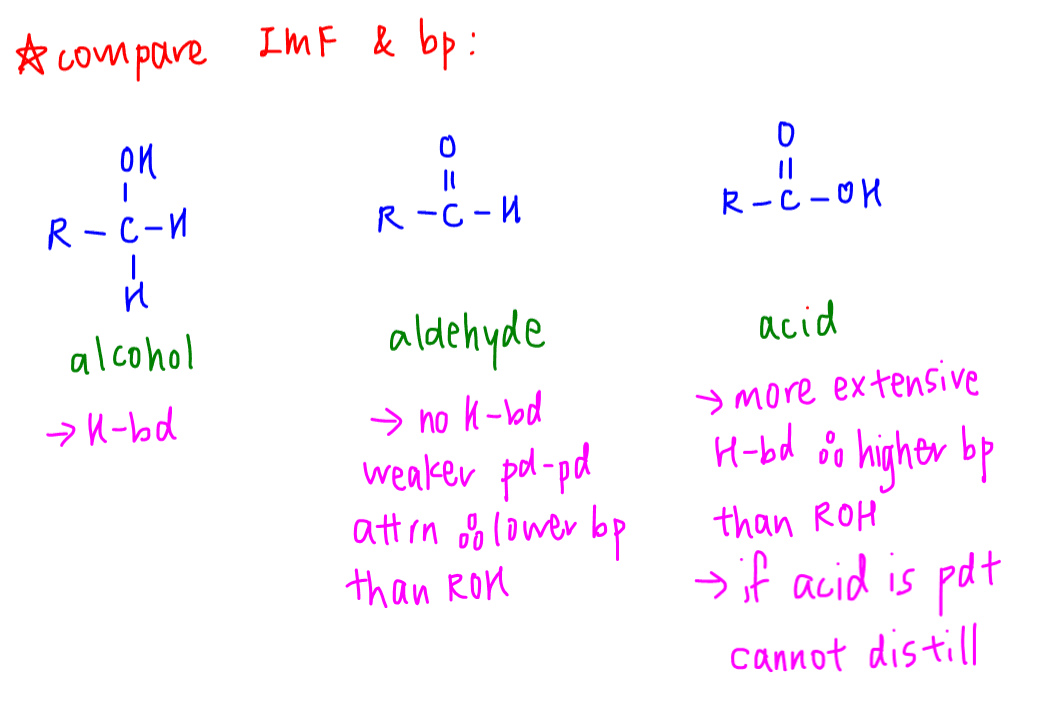
Using concepts from Chemical Bonding (Intermolecular Forces), we can deduce that:
- Alcohol can form hydrogen bonds
- Aldehyde cannot form hydrogen bonds so will have weaker permanent dipole - permanent dipole attractions, hence lower boiling point
- Acid has more extensive hydrogen bonds due to additional acyl C=O group, hence higher boiling point than alcohol
If we were to oxidise primary alcohol to acid, we cannot remove the acid via distillation since it has a higher boiling point than alcohol.
Therefore in order to remove product via distillation, we have to ensure that the product has a lower boiling point than its reactant.
Topic: Alcohols, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's trusted JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
