Determine pH of Resultant Solution of Acid-Base Reaction
Let Chemistry Guru, Singapore's renowned JC Chemistry tuition centre, guide you through this question.
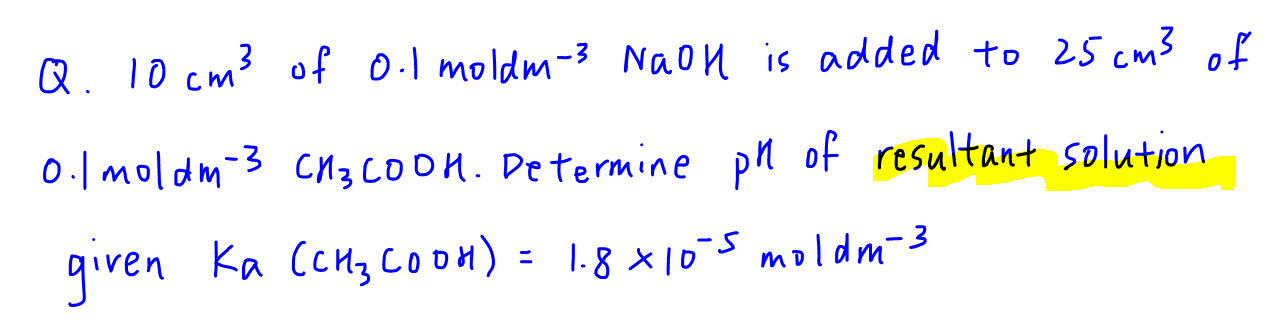
When there is an acid base reaction, the first and most important thing is to determine the resultant solution.
Once we know the resultant solution, the method to determine its pH will be obvious.
1. Determine Resultant Solution
Personally I like to use the ICE table to work out the reaction and determine the resultant solution.
ICE table is a tabulated presentation of mole concept calculations.
In 3 rows we can determine the amount of reactants and products left at the end of the reaction, ie the resultant solution.
I - initial amount of reactants and products
C - change in the amount of reactants and products (follow the mole ratio of balanced equation)
E - amount of reactants and products at the end of the reaction
Therefore ICE table is a very concise way to present and figure out the resultant solution of any reaction.
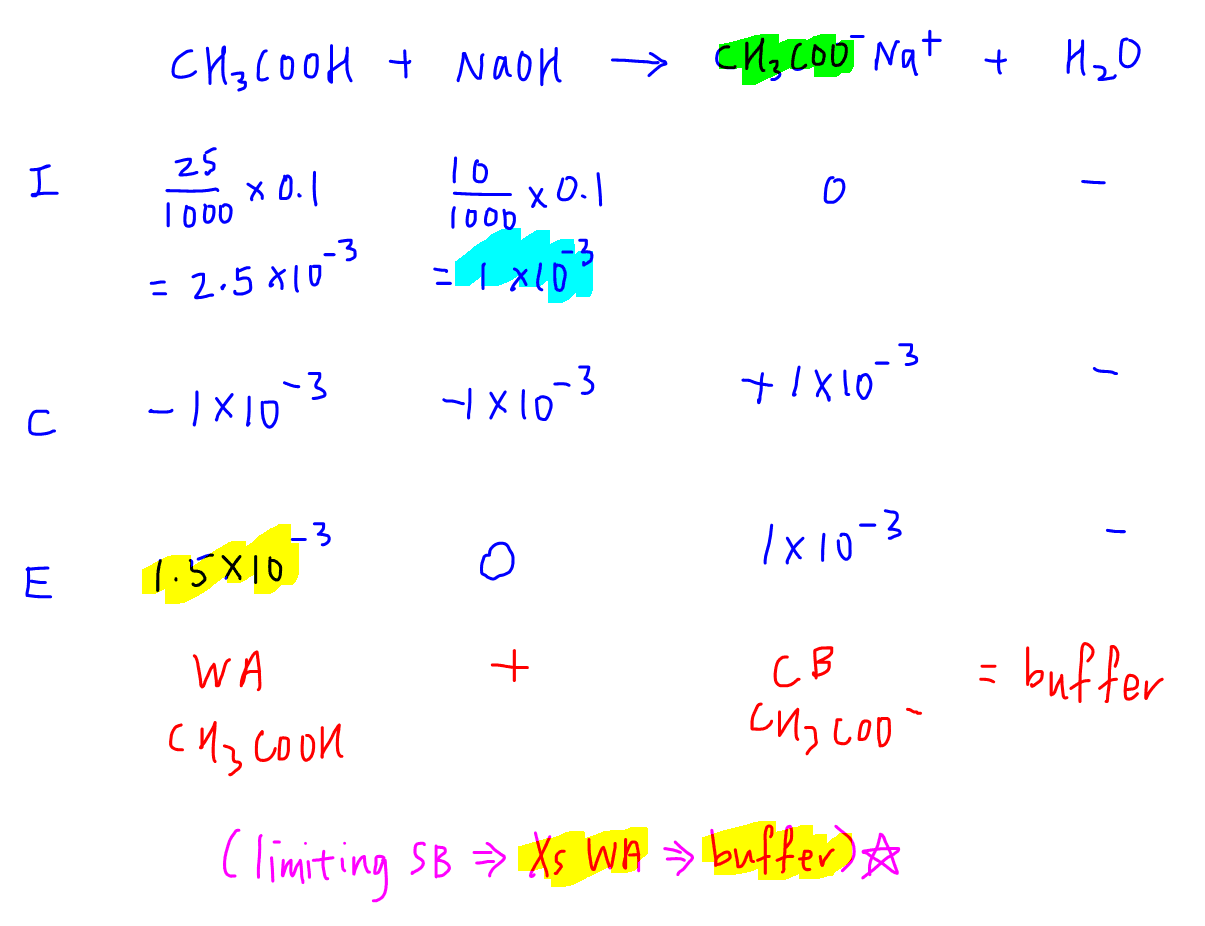
For this question, we notice weak acid CH3COOH is in excess, hence the resultant solution will be a mixture of weak acid CH3COOH and salt CH3COO-Na+.
2. Deduce Nature of Resultant Solution
The salt CH3COO-Na+ is made up of conjugate base CH3COO- and neutral Na+, hence only CH3COO- will affect the pH of the solution.
If you are unclear about the concept on salt hydrolysis, do check out my previous video on this.
Therefore the resultant solution is a mixture of weak acid and its conjugate base, ie an acidic buffer.
I have a previous video on determining acidic and alkaline buffer solutions so do take a look if you are not clear about this.
3. Calculate pH of Resultant Solution
Since the resultant solution is a buffer solution, we can use the buffer equation to determine its pH.

Notice I've used the mole ratio of CH3COO- to CH3COOH instead of concentration ratio.
In a buffer solution, both conjugate acid-base pair are sharing the same volume, hence mole ratio is equal to concentration ratio.
For the in depth discussion for this exercise, check out this video now!
Topic: Buffer and Titration Curve, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online tuition classes!
