Position of Substitution of Benzene Substituents
In Organic Chemistry we often have to consider whether a substituent attached to a functional group is an electron donating group or electron withdrawing group.
We need this concept to determine how this substituent interacts with the functional group and affects its stability and reactivity.
We actually learn this idea under the topic of Benzene where we determine the Position of Substitution of Benzene Substituents.
Before that let's go through these 2 concepts.
1. Position of Substitution (ortho, para, meta directing)
A substituent that is already attached to benzene will influence where the next incoming group goes to.
There are 2 ways the substituent can direct the incoming group:
a. 2,4 - directing (ortho para directing) : incoming group will be substituted at position 2 (ortho) or position 4 (para) with respect to the substituent.

b. 3 - directing (meta directing) : incoming group will be substituted at position 3 (meta) with respect to the substituent.

2. Reactivity of Benzene for Electrophilic Substitution (Electron donating groups and electron withdrawing groups)
a. Electron Donating Groups will increase electron density of benzene, make it more attractive to an electrophile, hence increase benzene's reactivity. Therefore electron donating groups are activating groups to benzene.

b. Electron Withdrawing Groups will decrease electron density of benzene, make it less attractive to an electrophile, hence benzene becomes less reactive. Hence electron withdrawing groups deactivate benzene.

How to Determine Position of Substitution and Electron Donating or Electron Withdrawing Group for Substituents?
This can be summarised in the table below:
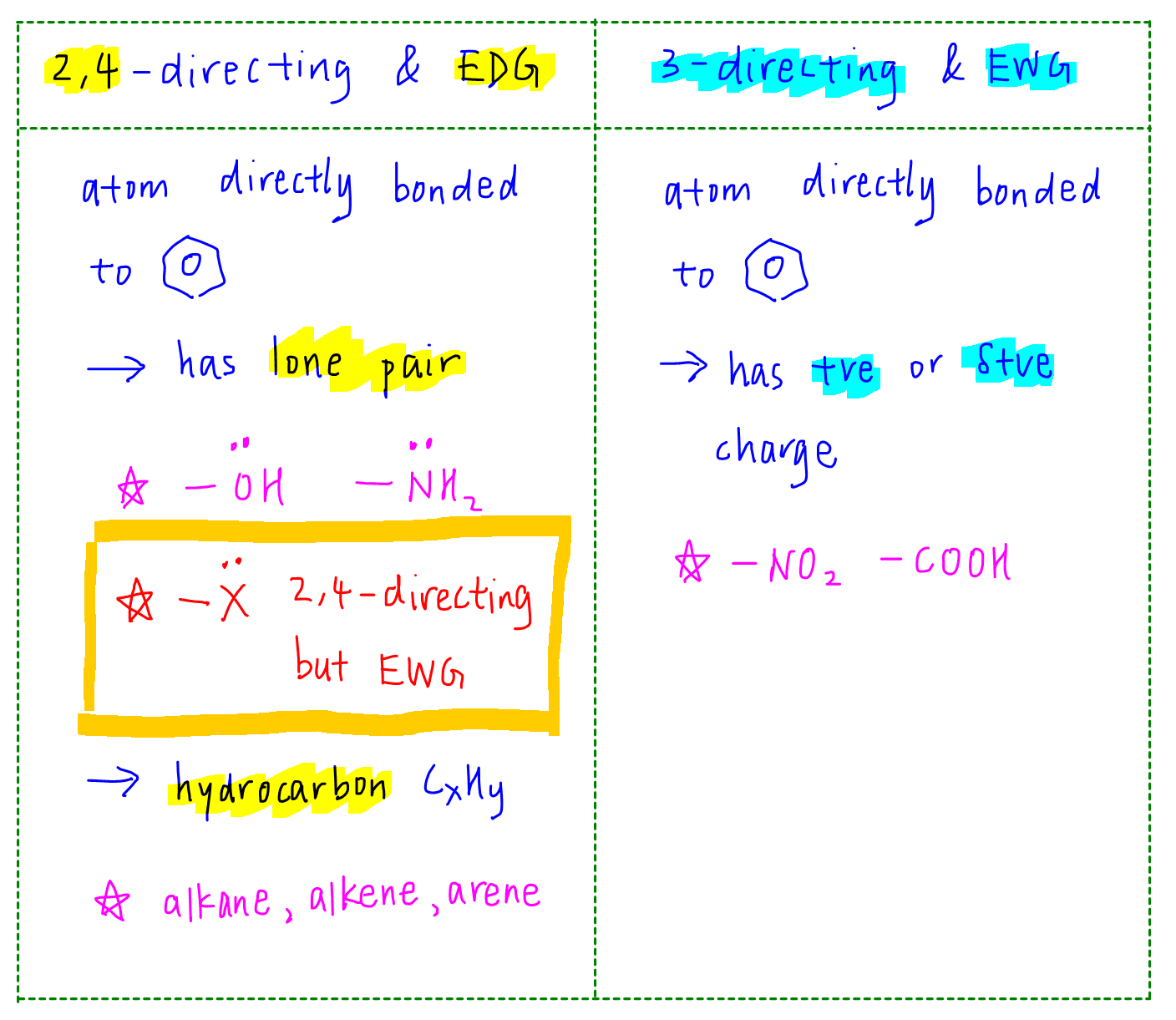
We focus on the atom directly attached to benzene. If that atom:
a. has a lone pair, it will be 2,4 - directing (ortho para directing) and electron donating. Exception is halogen which is 2,4 - directing but electron withdrawing.
b. is a hydrocarbon (CxHy), it will be 2,4 - directing and electron donating.
c. has a positive or partial positive charge, it will be 3 - directing (meta directing) and electron withdrawing.
Students will find it easier to remember these guidelines instead of memorizing a whole set of substituents under each category.
For the detailed step-by-step discussion on this topic, check out this video!
Topic: Benzene, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's reputable A Level Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online chemistry classes!
