Practical Qualitative Analysis
Since Practical Paper 4 is just round the corner, let's have a discussion involving Qualitative Analysis!
I would also like to wish all J2 students the very best of luck for your Practical exams!
QA is an integral part of Practical Exams, and are usually pretty straightforward.
We need to conduct the tests as instructed, write down the observations and deduce the identify of unknown species present in a solution.
Let's go through an exercise where the observations are already given (since the chemicals and experimental apparatus are not available), and deduce the unknown ions present in solution FB8.

It's recommended to keep track of the number of cations and anions to be identified, since each ion usually will be identified via one test only.
So in this case we have 2 cations and 1 anion to identify.
Test 1

We can refer to the Qualitative Analysis Notes at the end of the Practical Exam Paper or Data Booklet so there is no need to memorise the observations for all cations and anions.
So let's figure out which species will give green precipitate on adding aqueous ammonia.
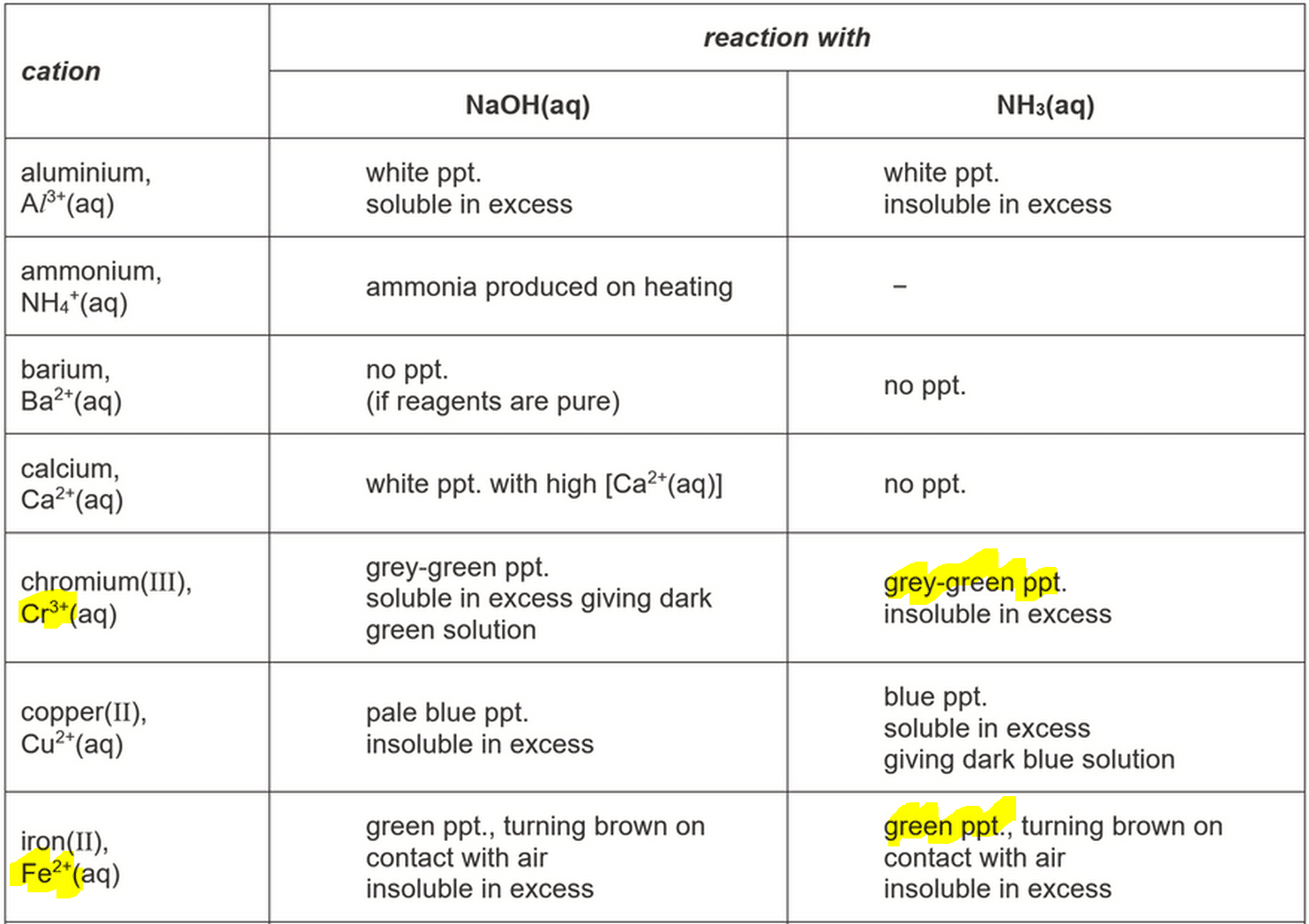
From (a) reaction of aqueous cations of QA Notes, we can see that only Cr3+ and Fe2+ will give green ppt, insoluble in excess ammonia.
However only Fe2+ will form green ppt that turns brown on contact with air.
Hence Fe2+ is present, which forms green Fe(OH)2 ppt on adding aqueous ammonia, insoluble in excess.
Fe(OH)2 ppt is oxidised to brown Fe(OH)3 ppt on standing in air.
Test 2

Green ppt formed on adding aqueous sodium hydroxide is due to Fe2+ as identified in Test 1.
On gentle heating colourless gas evolved that turns moist red litmus paper blue.
The colourless gas must be ammonia, which can be deduced from QA notes part (c).
Referring to QA notes part (a) we can determine the cation that gives this observation must be NH4+.
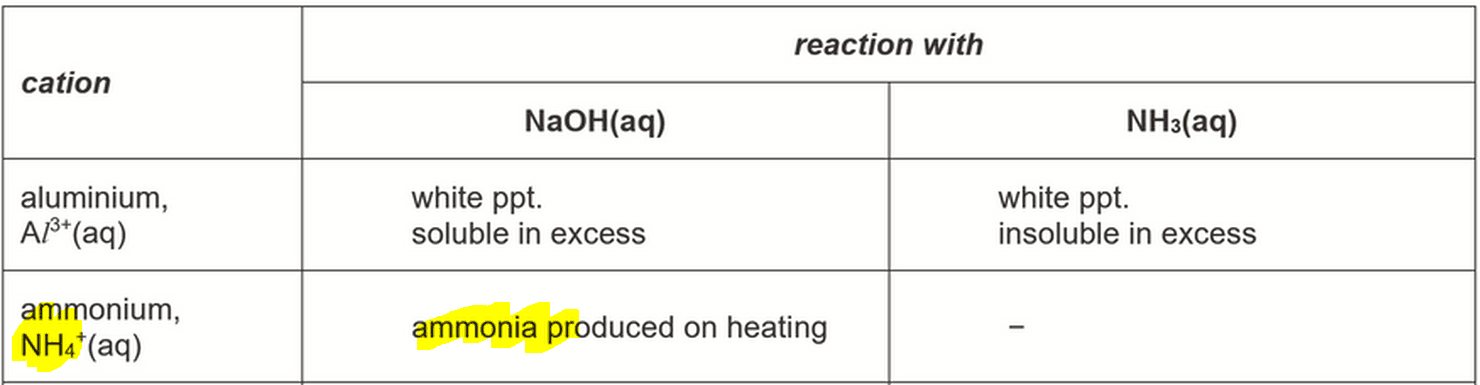
On adding strong base NaOH, neutralisation takes place between NaOH and weak acid NH4+ which forms NH3 and water.
The NH3 gas is then driven off on heating.
The last observation where green ppt turned brown is due to formation of Fe(OH)3.
Test 3

Brown ppt remained on adding H2O2 means there is no redox reaction between Fe(OH)3 and H2O2.
We can calculate the Ecell for this reaction to show that the redox reaction between Fe(OH)3 and H2O2 in alkaline medium is not feasible.
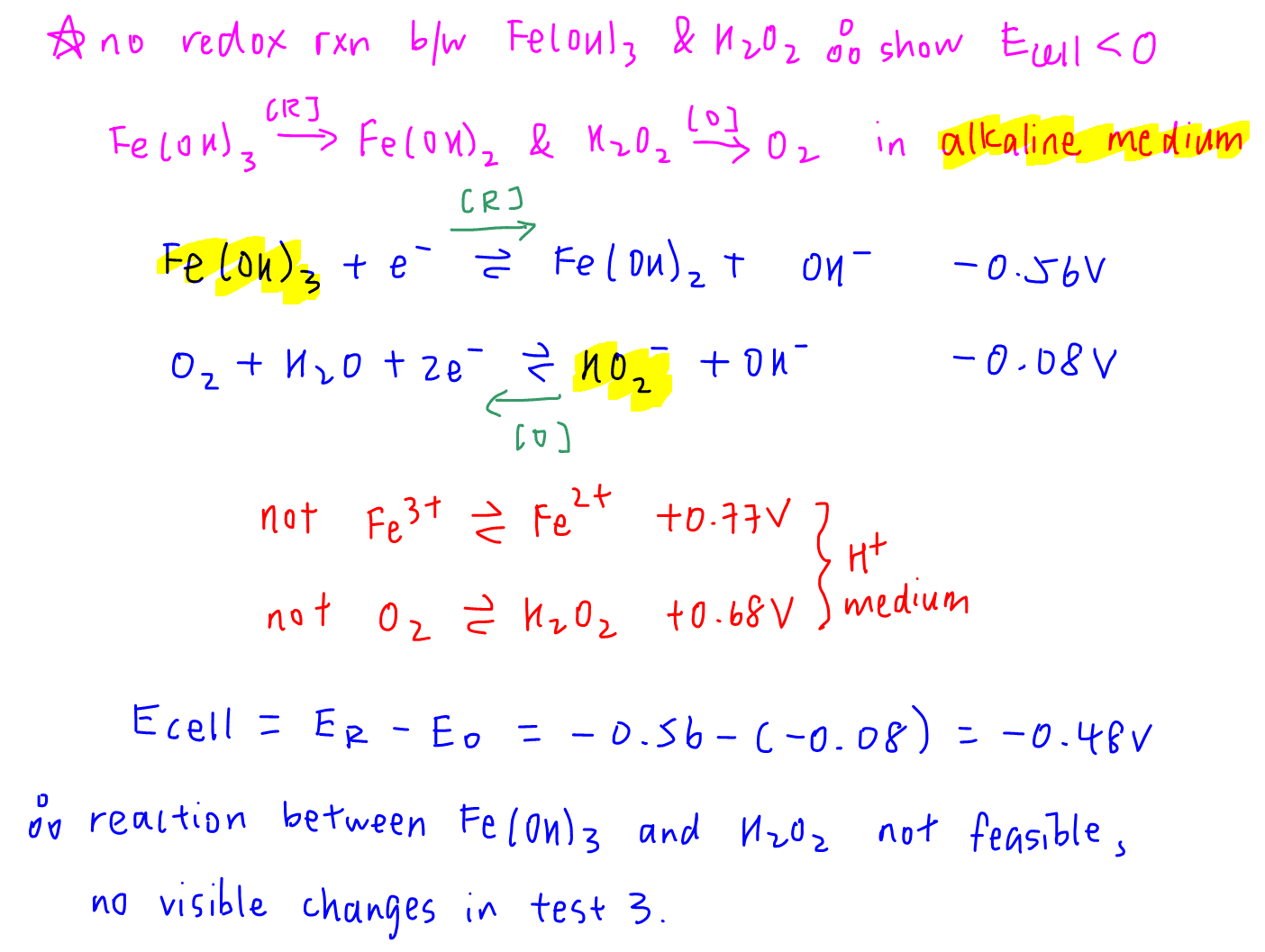
Test 4

Since both cations are determined, we know that Test 4 is for identification of the remaining anion.
From the QA Notes part (b), we can determine that both SO42- and SO32- give white ppt with Ba2+.
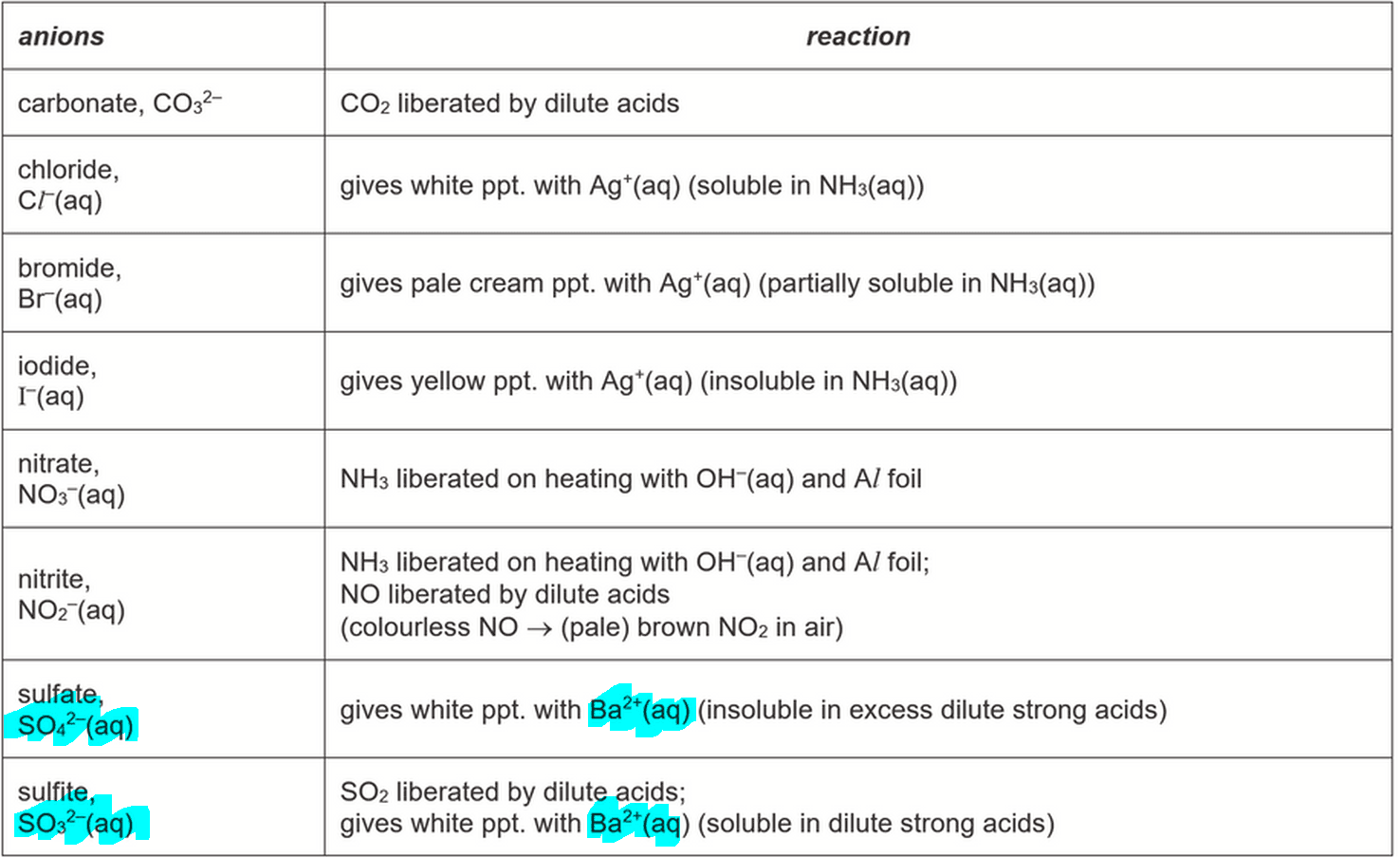
However only SO42- is insoluble in excess dilute HCl, hence the anion must be SO42-.
Ions present in FB8
Finally we have determined all the ions present in solution FB8
Cations: Fe2+, NH4+
Anion: SO42-
Topic: Inorganic Chemistry, A Level Chemistry, Singapore
Back to other previous Inorganic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online tuition classes!
