Pseudo First Order Reactions - Kinetics
In this video we want to discuss the concept of Pseudo first order reaction in Kinetics.
Let Chemistry Guru, Singapore's top JC Chemistry tuition centre, guide you through an example:
Given a reaction is first order with respect to both reactants A and B.
For an experiment, where [A] = 0.01 moldm-3 and [B] = 0.50 moldm-3, the half life was determined to be 10 minutes.
What is the new half life when the concentration of B is changed to 1.00 moldm-3?
1. Determine rate equation
Let's first write down the rate equation based on the order of the reaction with respect to A and B.
Since order is 1 with respect to both reactants then the rate equation is given as:
rate = k [A][B]
The overall order of the reaction is 2, which makes it difficult for us to determine half life as we know that half life is not constant for overall order 2 reaction.
In fact, in A Level Chemistry syllabus we are only required to find half life for first order reactions.
This means that the question is hinting to us that in order for us to determine half life for a second order reaction, we need to change this reaction into a first order reaction.
That's where the concept of pseudo first order reaction comes in.
2. Criteria for Pseudo First Order Reaction
Once we suspect the question is about pseudo first order reaction we can verify this by looking out for one of these criteria:
a. excess reactant - the concentration of excess reactant is treated as constant since the change in the amount of excess reactant is negligible as the reaction proceeds.
b. catalyst - the concentration of catalyst is constant as it is chemically unchanged at the end of the reaction.
For this question we notice that the concentration of B is 50 times more than concentration of A.
So we can treat the concentration of B as constant and make it part of rate constant k.
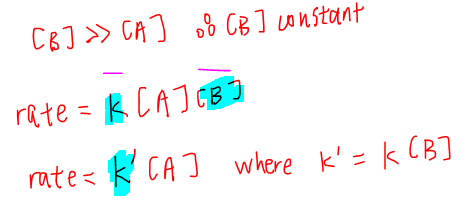
So what we have effectively done is to change an overall order 2 reaction and make it appear like an order 1 reaction.
Hence the name "pseudo first order reaction".
3. Determine Half Life
Since now the reaction is first order, half life is a constant and we can write it in terms of the original rate constant k and concentration of B.
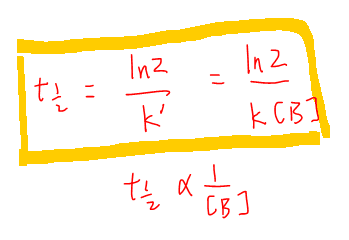
From the expression we know that half life is inversely proportionate to concentration of B.
Comparing the 2 experiments, when we double the concentration of B from 0.5 moldm-3 to 1.0 moldm-3, we will expect the half life to be halved from 10 minutes to 5 minutes.
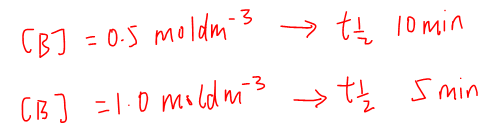
For the detailed step-by-step discussion on how to apply Pseudo first order reaction concept, check out this video!
Topic: Kinetics, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's leading JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online chemistry classes!
