Rate Equation and Order of Reaction
For a general reaction:
xA + yB → products
The rate equation can be expressed as the following:
rate = k[A]m [B]n
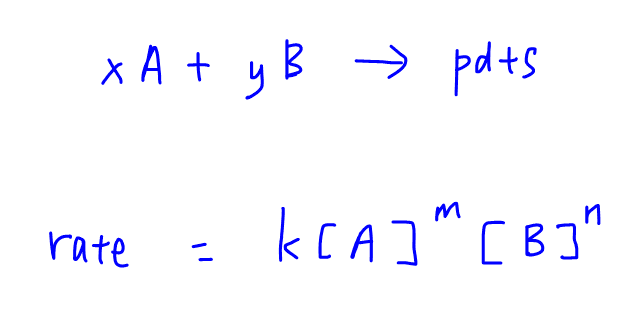
k is rate constant which is affected by temperature and activation energy.
When temperature increases, k will increase.
When a catalyst is used and activation energy is lowered, k will increase.
m and n are the order of reactions with respect to A and B respectively.
In A Level Chemistry syllabus, order of reaction is restricted to order 0, 1 and 2.
Therefore we need to be familiar with the rate equation and concentration time graph for the various orders.
Zero Order with respect to reactant A
When order is zero, rate equation is:
rate = k [A]0 = k
This means rate is independent of concentration of A and will remain constant throughout the reaction.
In the concentration time graph, the rate of the reaction is given by the gradient of the graph.
So the expected concentration time graph for zero order reaction will be a straight line.

First Order with respect to reactant A
When order is 1, rate equation is:
rate = k [A]
Rate is directly proportionate to concentration of A.
The concentration time graph will be a curve.
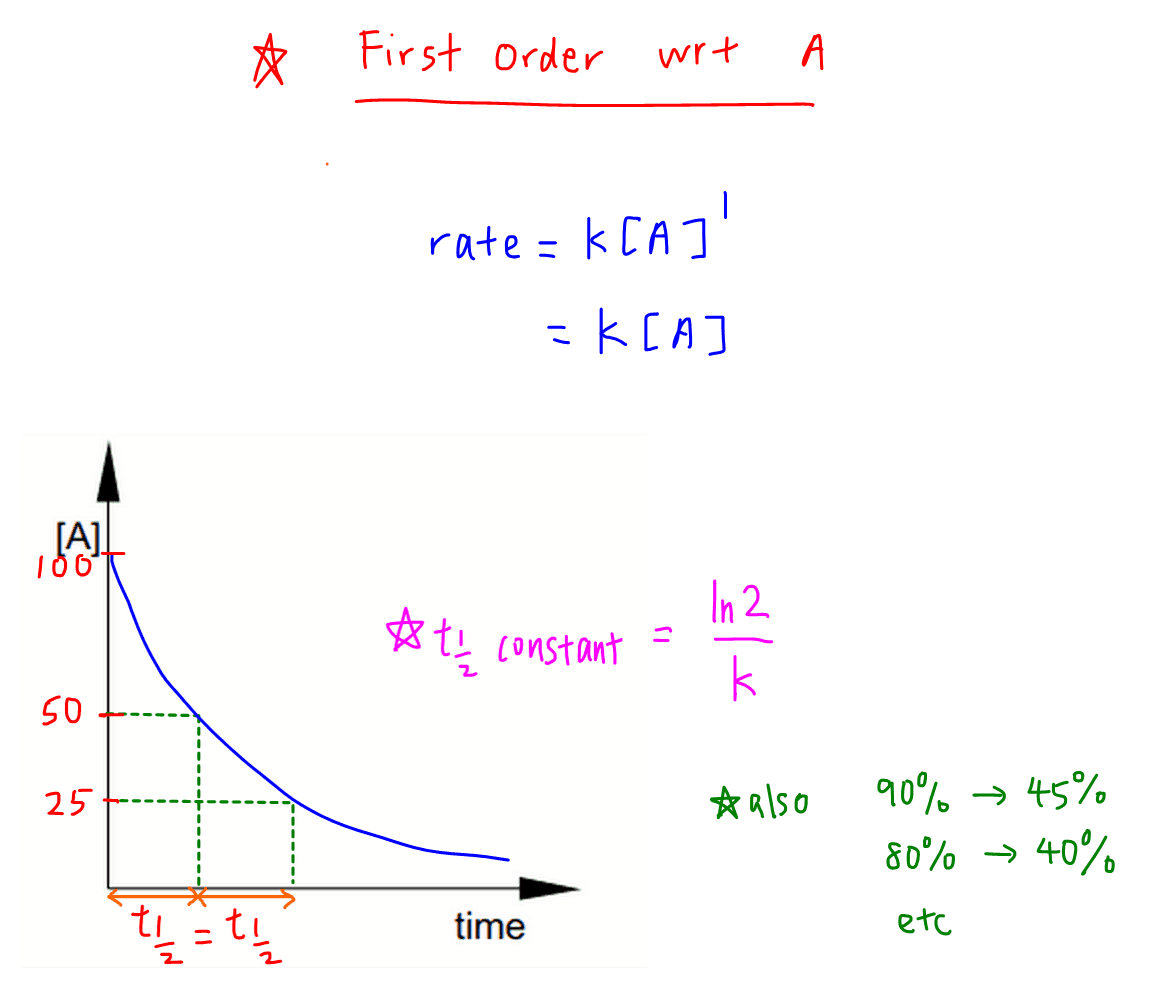
Half life is the time taken to decrease the concentration of a reactant to half of its original amount.
The half life of a first order reaction is a constant, and is related to rate constant via the following formula:
thalf = ln 2 / k
Usually we will use the following concentrations to determine half life:
100% to 50% reactant
50% to 25% reactant
But we can use any concentration of reactant and time taken to half that amount will still be half life:
90% to 45% reactant
80% to 40% reactant
etc
Second Order with respect to reactant A
When order is 2, rate equation is:
rate = k [A]2
The concentration time graph will be a curve which is similar to first order reaction.

Half life of second order reaction is not a constant, so we can use this to differentiate second order reaction from first order reaction.
Determine Order from Concentration Time Graph
In a kinetics experiment, the data collected will be concentration of reactant or product (or properties that are related to them such as volume, colour intensity, etc) and time.
Hence the first graph plotted will always be concentration time graph.
From the shape of the graph and half life, we can determine order of the reaction easily.
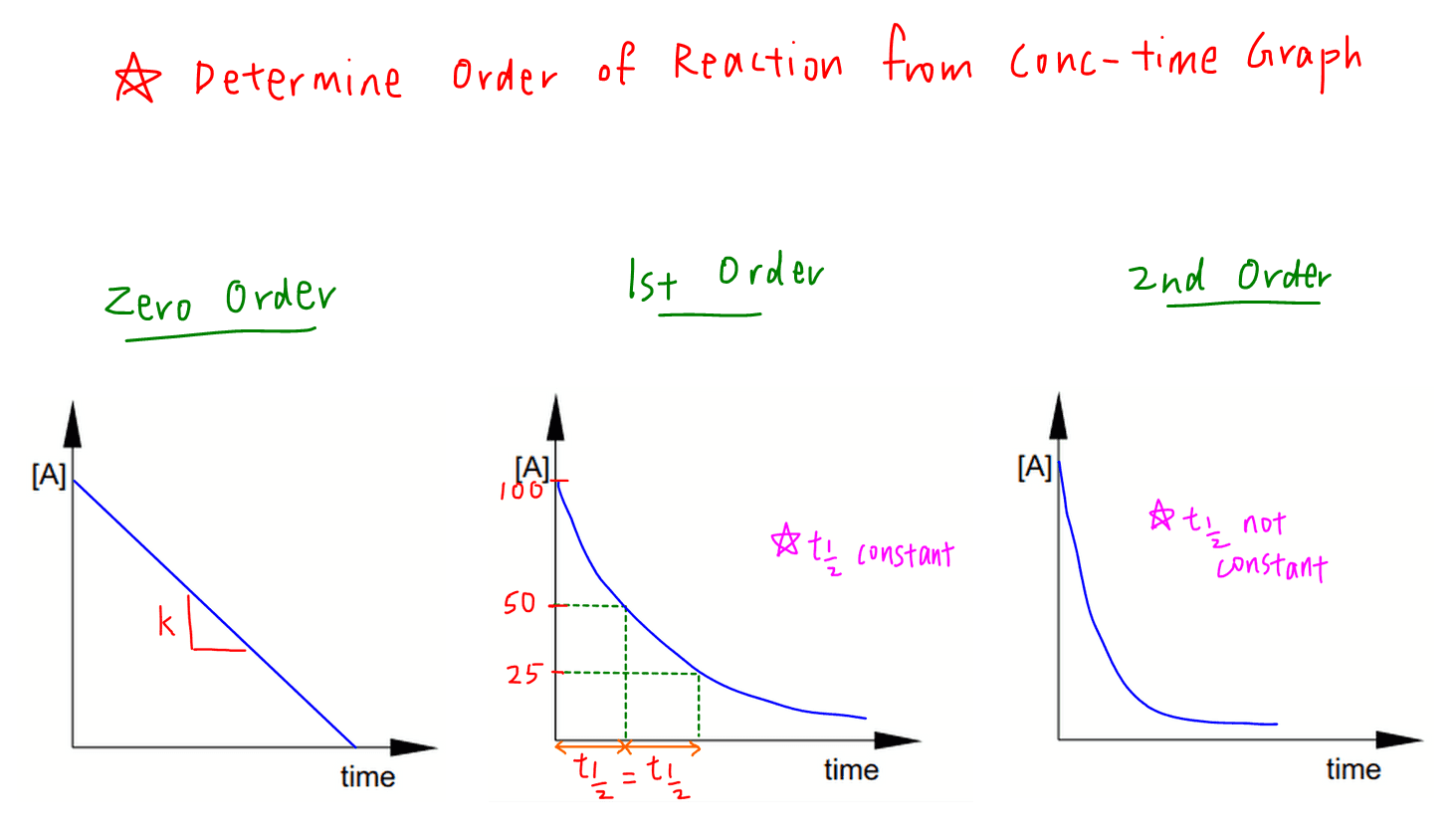
If concentration time graph is a straight line, order of reaction is zero.
If concentration time graph is a curve, compare half life.
If half life is constant, order of reaction is 1.
If half life is not constant, order of reaction is 2.
We can also determine order of reaction via the initial rates method.
Topic: Kinetics, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's renowned A Level Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online tuition classes!
