Reaction of Sodium with Organic Compounds
Let Chemistry Guru, Singapore's renowned JC Chemistry tuition centre, guide you through this question:
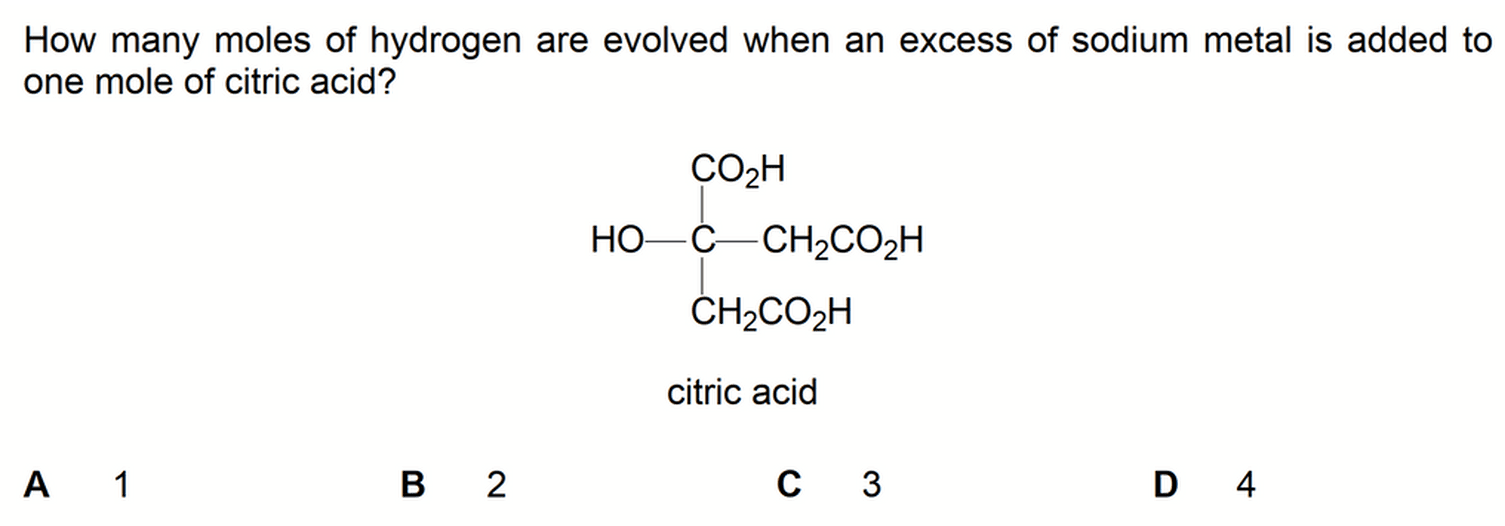
To determine the moles of hydrogen evolved we need to know the 3 functional groups that will react with sodium metal.
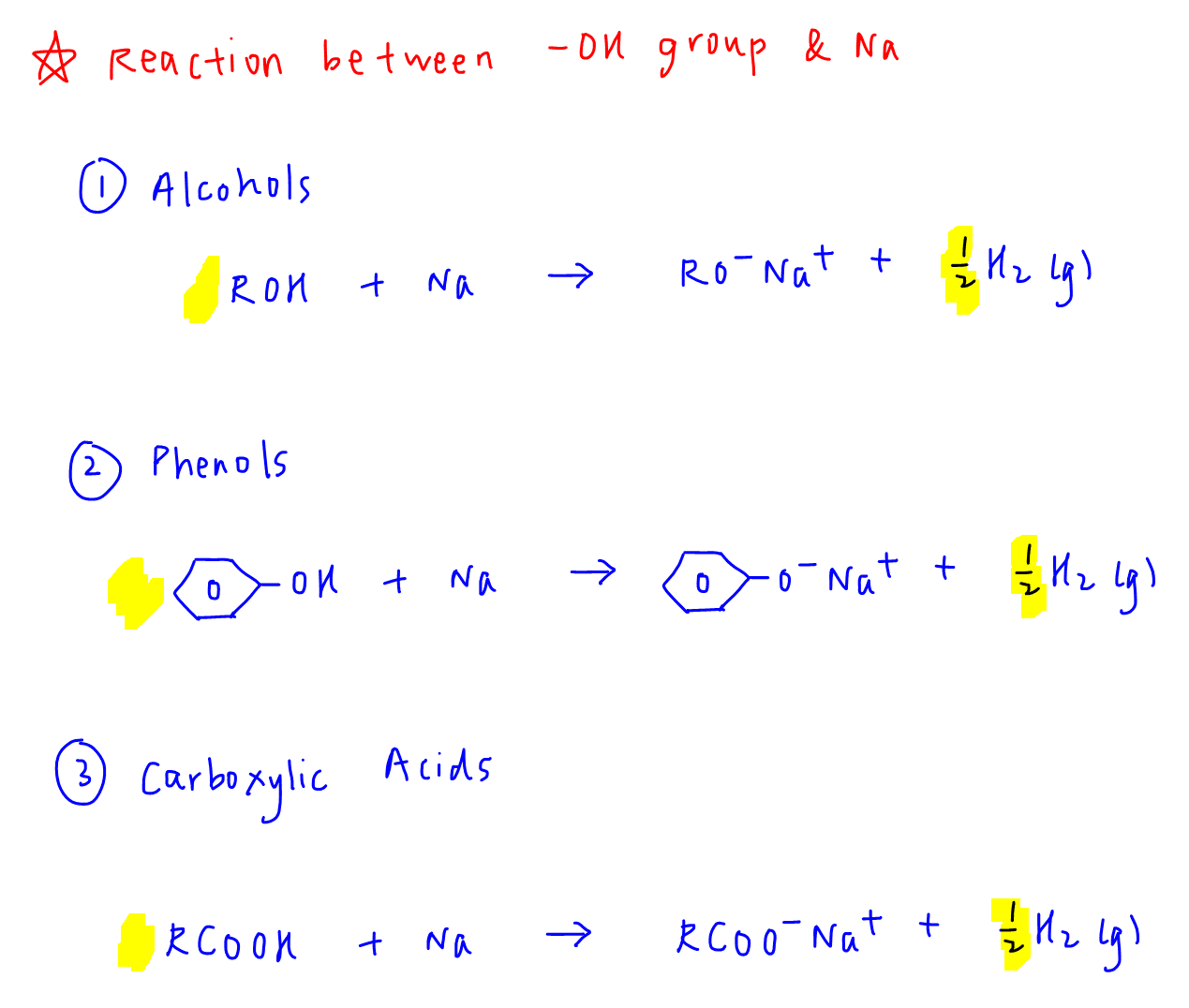
1. Alcohols to form alkoxide
ROH + Na → RO-Na+ + 1/2 H2(g)
2. Phenols to form phenoxide
PhOH + Na → PhO-Na+ + 1/2 H2(g)
3. Carboxylic acids to form carboxylate
RCOOH + Na → RCOO-Na+ + 1/2 H2(g)
All these functional groups undergo deprotonation or donate their proton.
Hence the reaction with sodium is considered as acid-base or neutralisation with respect to organic compound.
Here's my video lesson on how to compare acidity of these organic compounds.
Finally we can now look back at citric acid.
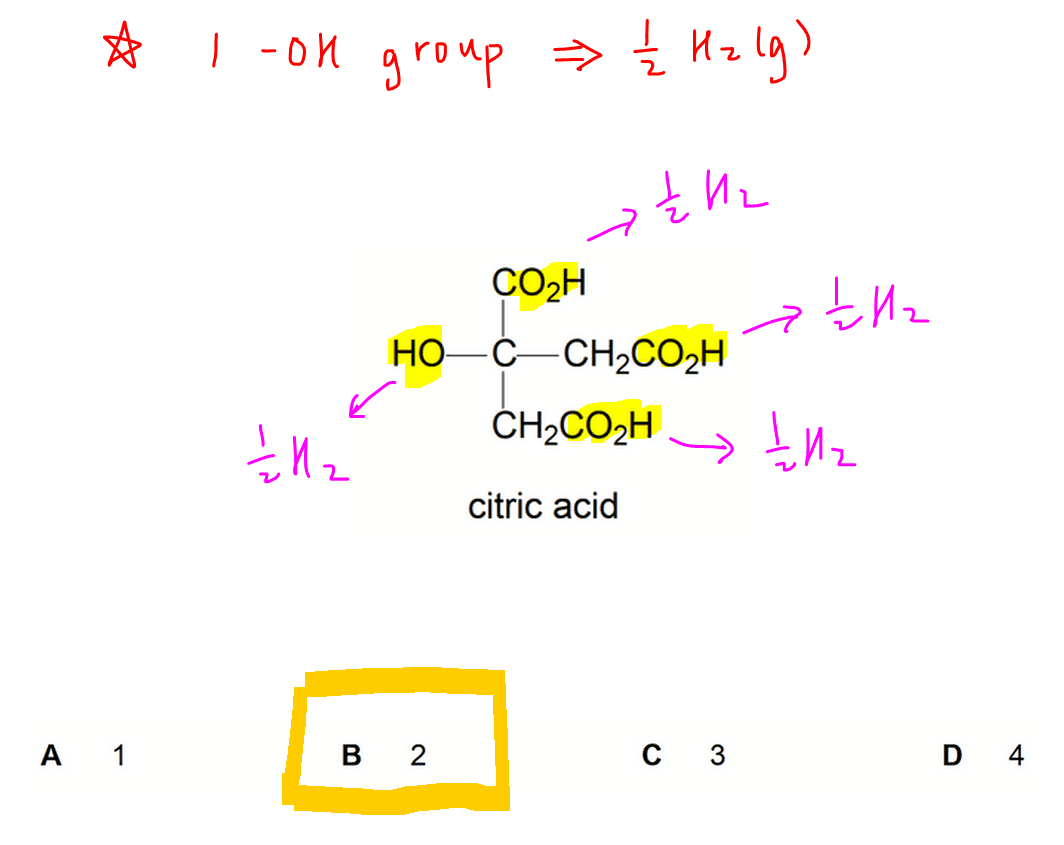
Citric acid contains 3 carboxylic acid groups and 1 alcohol group.
For each of these functional groups, one mol of functional group will form 0.5 mol of H2 gas.
Hence 1 mol citric acid will produce 2 mol H2(g). [Answer = B]
Topic: Carboxylic Acid, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online tuition classes!
