How to Draw Shapes of Orbitals
Let's learn about the shapes of atomic orbitals in this video lesson created by Chemistry Guru, Singapore's leading JC Chemistry tuition centre.
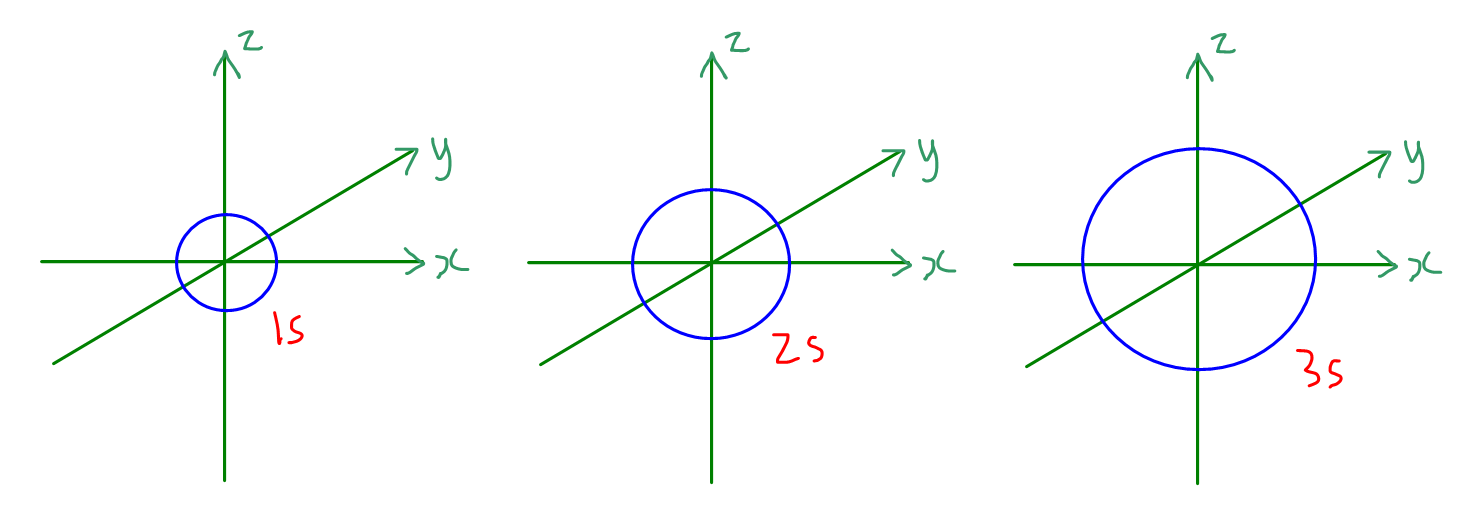
1. s orbitals
There is only 1 s orbital in each s subshell.
Shape of s orbital is spherical and non-directional.
Size of s orbital increases as principal quantum number n increases.
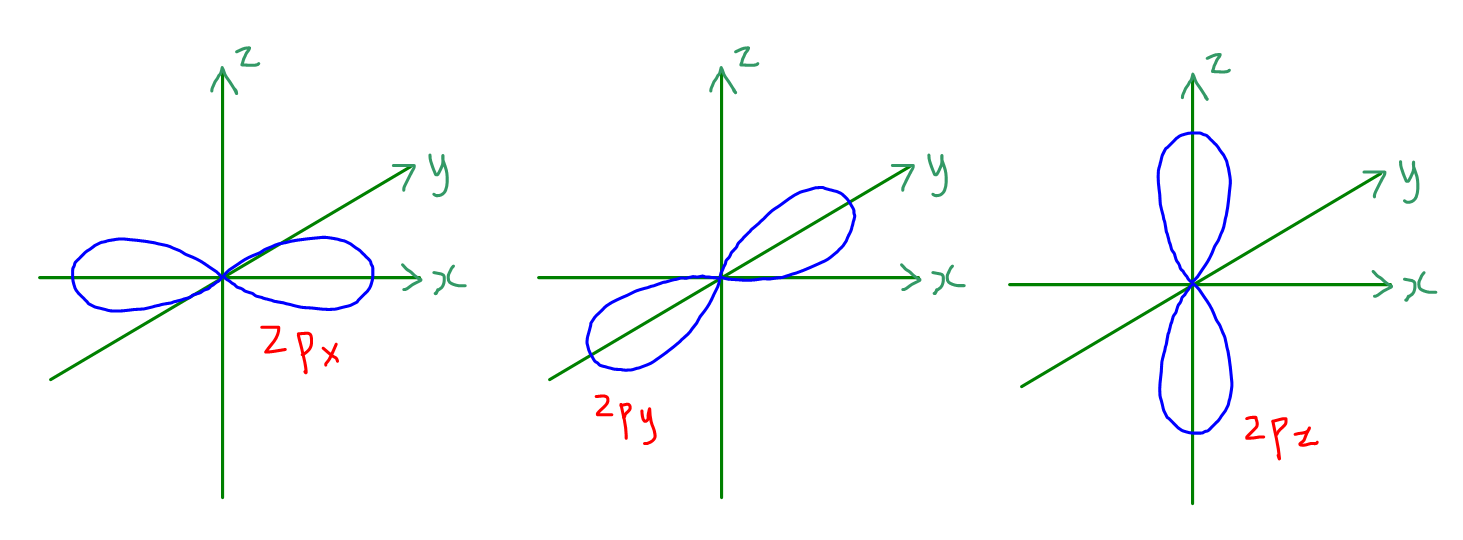
2. p orbitals
There are 3 p orbitals in each p subshell, namely npx, npy and npz orbitals.
The orbitals are dumb-bell shaped or look like the number 8 or infinity sign ∞.
npx lies along the x-axis, npy along the y-axis and npz along the z-axis.
Orbitals in the same subshell are degenerate or have the same energy.
Size of p orbital increases as n increases.
3. d orbitals
Each d subshell has 5 degenerate orbitals, namely ndxy, ndyz, ndxz, ndx2-y2 and ndz2
Shape of ndxy, ndyz, ndxz and ndx2-y2 is cloverleaf shape with 4 loops or butterfly shaped.
Shape of ndz2 is dumb-bell shape with a ring around its centre.
ndxy lies on the xy plane, ndxz on the xz plane and ndyz on the yz plane, but they are not along the axis.
ndx2-y2 lies directly along the x and y axis.
ndz2 lies directly along the z axis.
Remember the orbitals with a square term (ndx2-y2 and ndz2) will be lying directly along the axis.

Topic: Atomic Structure, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
