Compare Solubility of Silver Phosphate in Different Solutions

The dissociation of silver phosphate and solubility product expression is written as follows:
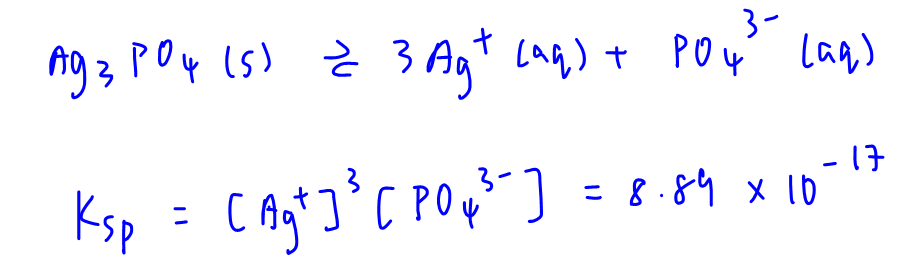
We need to calculate solubility of silver phosphate in different scenarios.
Let x be the solubility in each case.
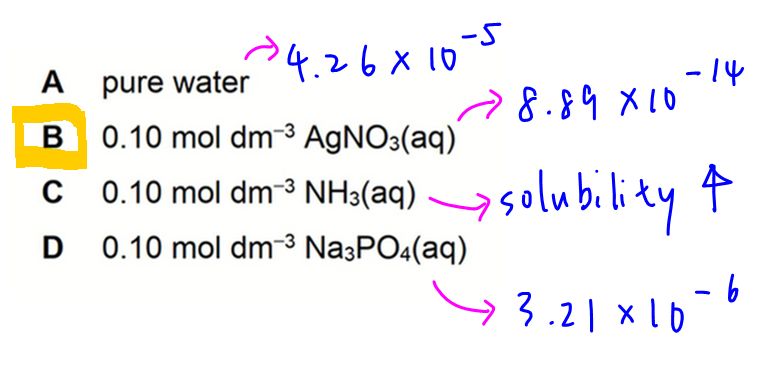
A. Pure water
This is a straightforward dissolving of silver phosphate to give a saturated solution.
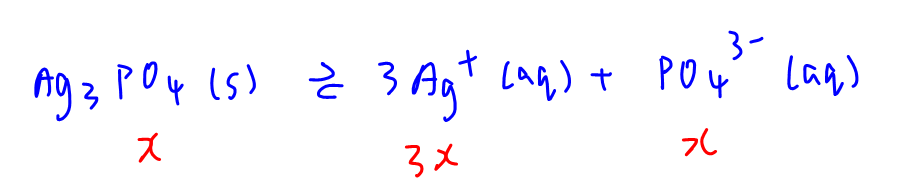
[Ag+] = 3x and [PO43-] = x
We can substitute these into Ksp expression and solve for x.
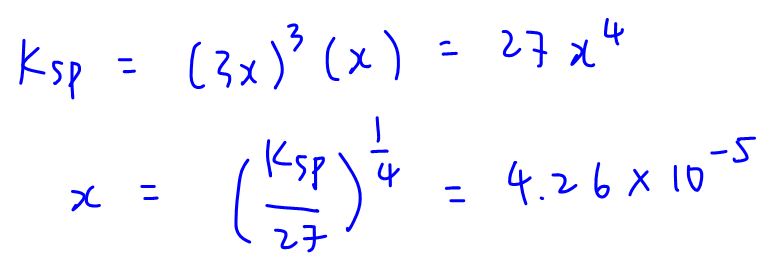
Solubility x = 4.26 x 10-5
B. 0.10 mol dm-3 AgNO3
AgNO3 will also contribute Ag+ into solution hence Ag+ is the common ion.
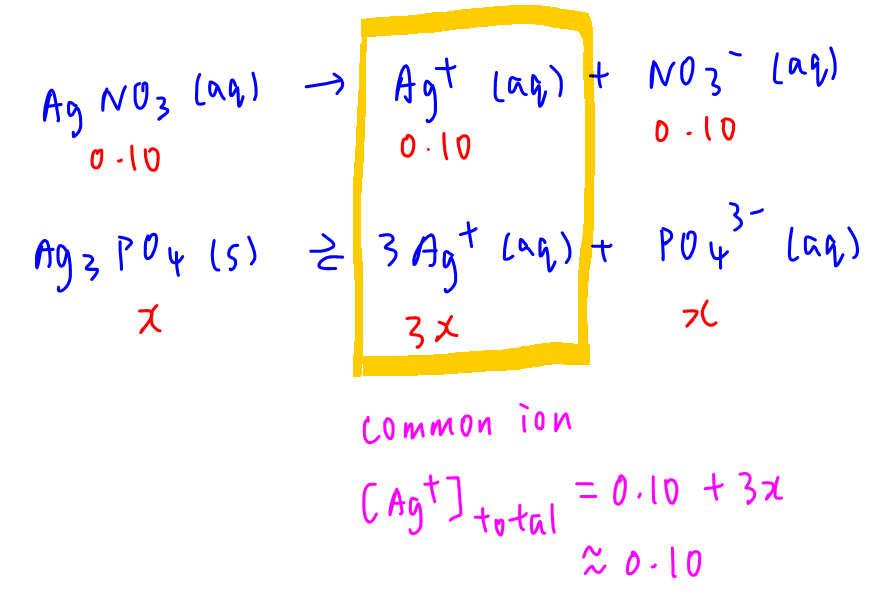
Total [Ag+] is approximated to 0.10 mol dm-3 and [PO43-] = x
Substitute into Ksp to solve for x.
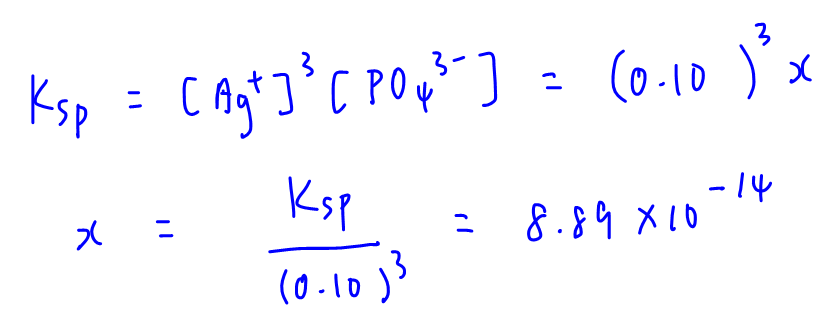
Solubility x = 8.89 x 10-14
Notice presence of common ion will suppress solubility. This is also known as the common ion effect.
C. 0.10 mol dm-3 NH3
Ammonia will form diammine silver complex with Ag+.
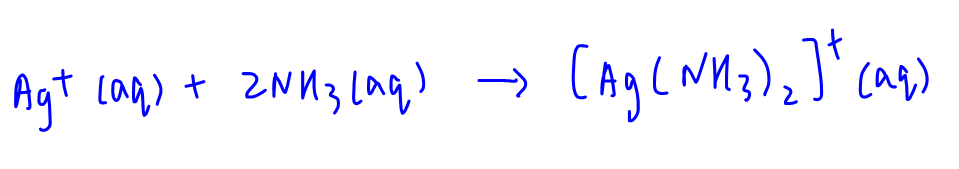
[Ag+] will decrease and POE for dissociation of Ag3PO4 will shift to the right.
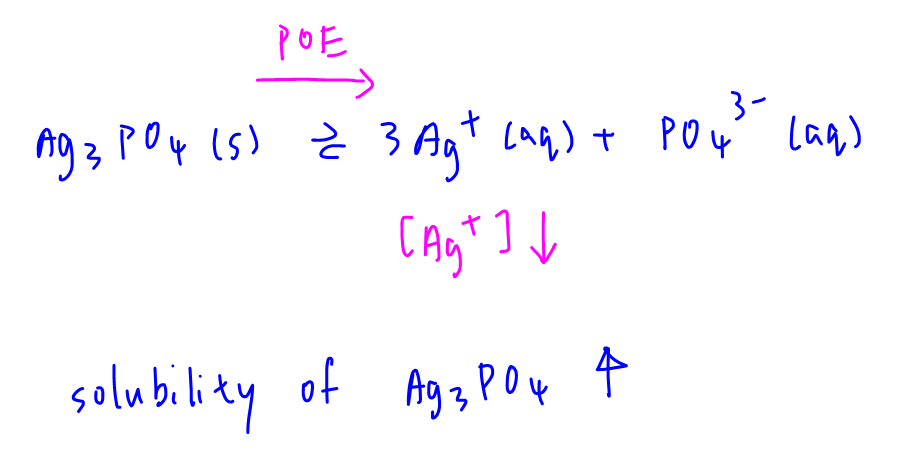
Hence solubility will increase.
D. 0.10 mol dm-3 Na3PO4
Na3PO4 will contribute PO43- into solution so PO43- is the common ion in this case.
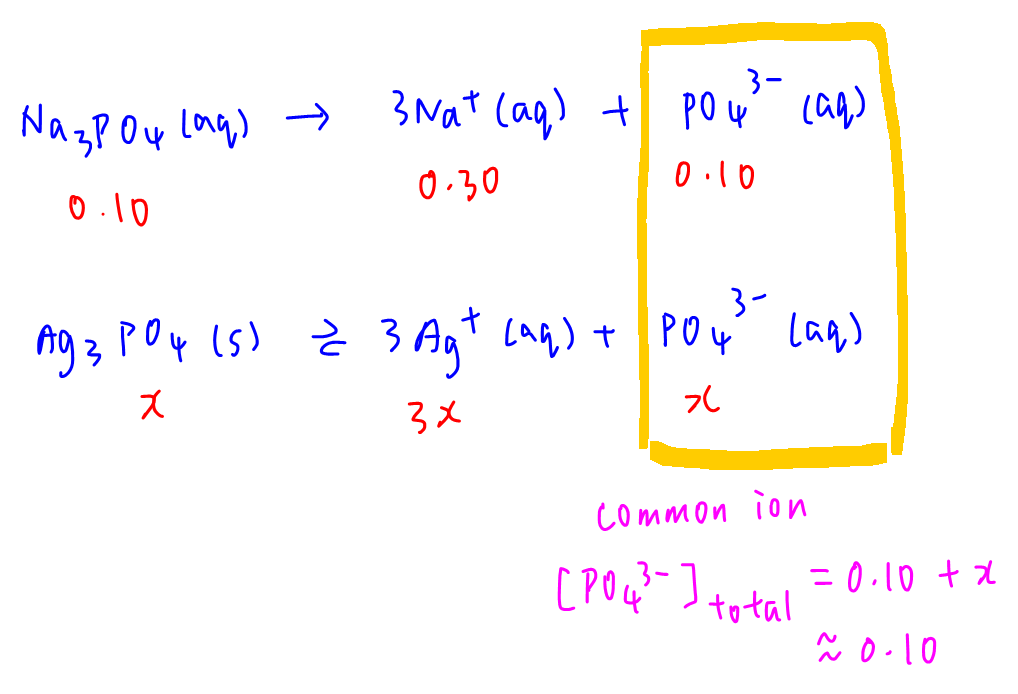
[Ag+] = 3x and total [PO43-] is approximated to 0.10 mol dm-3.
Substitute into Ksp to solve for x.
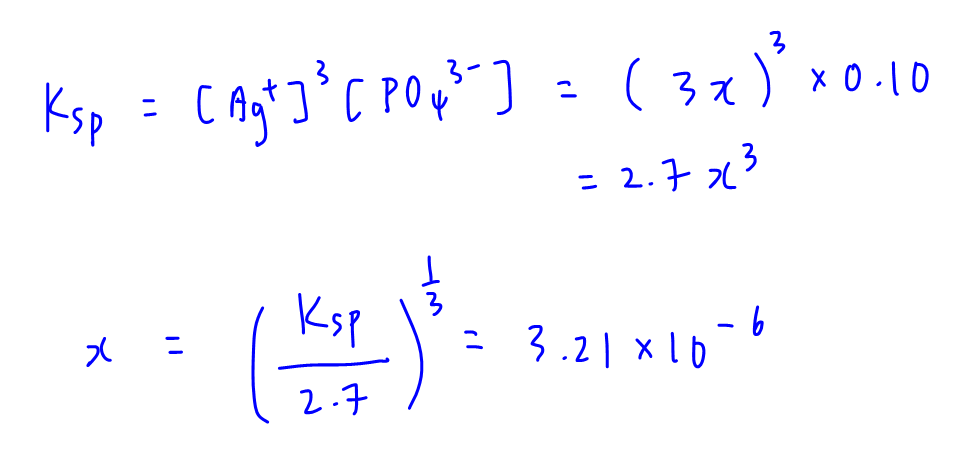
Solubility x = 3.21 x 10-6
Finally we can compare our answers to determine the lowest solubility which is option B.
Topic: Solubility Product, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's prestigious JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
