Singapore GCE A Level H1 Chemistry Syllabus 8873
Disclaimer: This is the web version of Singapore–Cambridge GCE Advanced Level H1 Chemistry (Syllabus 8873) for viewers who prefer this to the pdf format, painstakingly reproduced by Chemistry Guru, Singapore's top JC Chemistry tuition centre. Minor adjustments are made for alignment and easier web navigation.
To access the original pdf file from SEAB website please click here.
CONTENTS
SUBJECT CONTENT
3. Theories of Acids and Bases
5. The Mole Concept and Stoichiometry
SUMMARY OF KEY QUANTITIES AND UNITS
INTRODUCTION
Candidates will be assumed to have knowledge and understanding of Chemistry at O-Level, as a single subject or as part of a balanced science course.
This syllabus is designed to place less emphasis on factual material and greater emphasis on the understanding and application of scientific concepts and principles. This approach has been adopted in recognition of the need for students to develop skills that will be of long term value in an increasingly technological world rather than focusing on large quantities of factual material which may have only short term relevance.
Experimental work is an important component and should underpin the teaching and learning of Chemistry.
AIMS
The aims of a course based on this syllabus should be to:
1. provide students with an experience that develops interest in Chemistry and builds the knowledge, skills and attitudes necessary for them to become scientifically literate citizens who are well-prepared for the challenges of the 21st century
2. develop in students the understanding, skills, ethics and attitudes relevant to the Practices of Science, including the following:
2.1 understanding the nature of scientific knowledge
2.2 demonstrating science inquiry skills
2.3 relating science and society
3. develop the way of thinking to explain phenomena, approach and solve problems in chemical systems which involves students in:
3.1 understanding the structure, properties and transformation of matter at the atomic/molecular level and how they are related to each other
3.2 connecting between the submicroscopic, macroscopic and symbolic levels of representations in explaining and making predictions about chemical systems, structures and properties.
PRACTICES OF SCIENCE
Science as a discipline is more than the acquisition of a body of knowledge (e.g. scientific facts, concepts, laws, and theories); it is a way of knowing and doing. It includes an understanding of the nature of scientific knowledge and how this knowledge is generated, established and communicated. Scientists rely on a set of established procedures and practices associated with scientific inquiry to gather evidence and test their ideas on how the natural world works. However, there is no single method and the real process of science is often complex and iterative, following many different paths. While science is powerful, generating knowledge that forms the basis for many technological feats and innovations, it has limitations.
The Practices of Science are explicitly articulated in this syllabus to allow teachers to embed them as learning objectives in their lessons. Students’ understanding of the nature and limitations of science and scientific inquiry are developed effectively when the practices are taught in the context of relevant science content. Attitudes relevant to science such as inquisitiveness, concern for accuracy and precision, objectivity, integrity and perseverance should be emphasised in the teaching of these practices where appropriate. For example, students learning science should be introduced to the use of technology as an aid in practical work or as a tool for the interpretation of experimental and theoretical results.
The Practices of Science comprise three components:
1. Understanding the Nature of Scientific Knowledge
1.1 Understand that science is an evidence-based, model-building enterprise concerned with the natural world
1.2 Understand that the use of both logic and creativity is required in the generation of scientific knowledge
1.3 Recognise that scientific knowledge is generated from consensus within the community of scientists through a process of critical debate and peer review
1.4 Understand that scientific knowledge is reliable and durable, yet subject to revision in the light of new evidence
2. Demonstrating Science Inquiry Skills
2.1 Identify scientific problems, observe phenomena and pose scientific questions/hypotheses
2.2 Plan and conduct investigations by selecting the appropriate experimental procedures, apparatus and materials, with due regard for accuracy, precision and safety
2.3 Obtain, organise and represent data in an appropriate manner
2.4 Analyse and interpret data
2.5 Construct explanations based on evidence and justify these explanations through reasoning and logical argument
2.6 Use appropriate models1 to explain concepts, solve problems and make predictions
2.7 Make decisions based on evaluation of evidence, processes, claims and conclusions
2.8 Communicate scientific findings and information using appropriate language and terminology
3. Relating Science and Society
3.1 Recognise that the application of scientific knowledge to problem solving could be influenced by other considerations such as economic, social, environmental and ethical factors
3.2 Demonstrate an understanding of the benefits and risks associated with the application of science to society
3.3 Use scientific principles and reasoning to understand, analyse and evaluate real-world systems as well as to generate solutions for problem solving
1 A model is a representation of an idea, an object, a process or a system that is used to describe and explain phenomena that cannot be experienced directly. Models exist in different forms, from the concrete, such as physical scale models, to the abstract, such as diagrams or mathematical expressions. The use of models involves the understanding that all models contain approximations and assumptions limiting their validity and predictive power.
CURRICULUM FRAMEWORK
The key features of the H1 Chemistry Curriculum comprise Core Ideas and Extension Topics, Practices of Science and Learning Experiences as illustrated in Fig. 1.
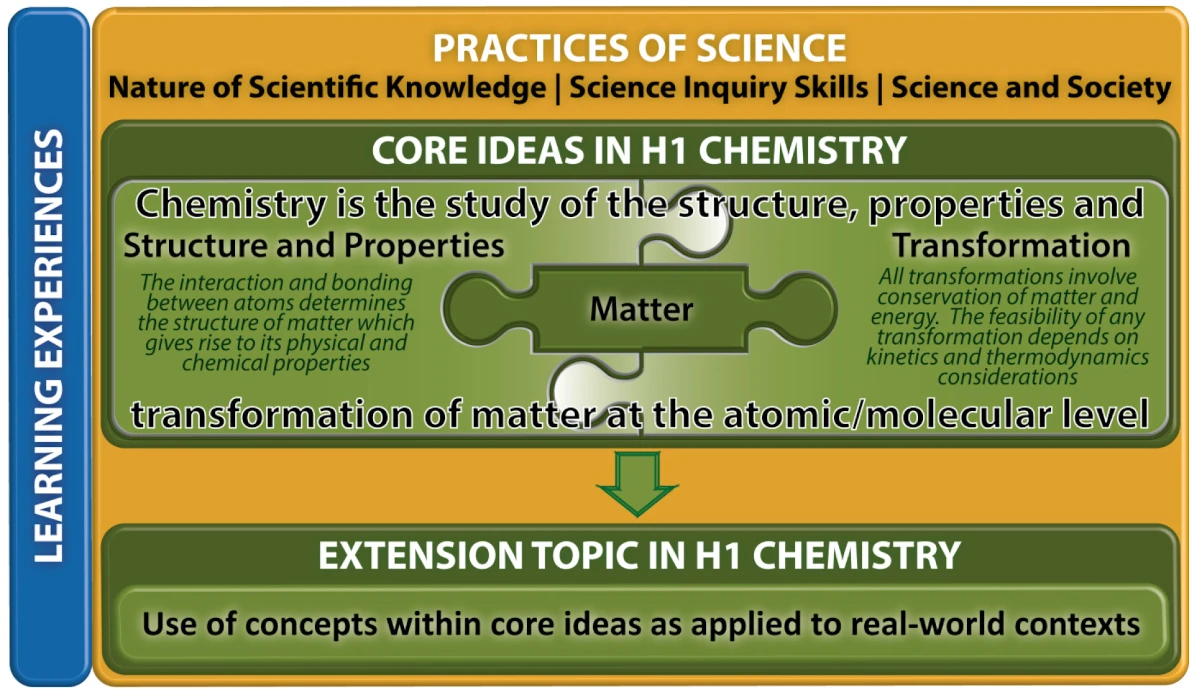
Fig. 1: H1 Chemistry Curriculum Framework
1. Core Ideas and Extension Topics
The topics in H1 Chemistry are organised as two levels underpinned by the Practices of Science:
(a) Core ideas: The three Core Ideas of Chemistry are Matter, Structure and Properties, and Transformation. The concepts in these Core Ideas are inter-related and form the basis for which further learning and understanding of chemical phenomena and reactions is built upon.
(b) Extension topics: Concepts in the Core Ideas are applied to real-world context in the study of nanomaterials and polymers.
2. Practices of Science
The Practices of Science are common to the natural sciences of physics, chemistry and biology. These practices highlight the ways of thinking and doing that are inherent in the scientific approach, with the aim of equipping students with the understanding, skills, and attitudes shared by the scientific disciplines, including an appropriate approach to ethical issues.
3. Learning Experiences
The Learning Experiences2 refer to a range of learning opportunities selected by teachers to link the chemistry content with the Core Ideas and the Practices of Science to enhance students’ learning of the concepts. Rather than being mandatory, teachers are encouraged to incorporate Learning Experiences that match the interests and abilities of their students and provide opportunities to illustrate and exemplify the Practices of Science, where appropriate. Real-world contexts can help illustrate the concepts in chemistry and their applications. Experimental activities and ICT tools can also be used to build students’ understanding.
2 The Learning Experiences can be found in the Teaching and Learning syllabus.
ASSESSMENT OBJECTIVES
The Assessment Objectives listed below reflect those parts of the Aims and Practices of Science that will be assessed.
A. Knowledge with understanding
Candidates should be able to demonstrate knowledge and understanding in relation to:
1. scientific phenomena, facts, laws, definitions, concepts and theories
2. scientific vocabulary, terminology and conventions (including symbols, quantities and units)
3. scientific instruments and apparatus, including techniques of operation and aspects of safety
4. scientific quantities and their determination
5. scientific and technological applications with their social, economic and environmental implications.
The syllabus content defines the factual knowledge that candidates may be required to recall and explain. Questions testing these objectives will often begin with one of the following words: define, state, name, describe, explain or outline (see the Glossary of Terms).
B. Handling, applying and evaluating information
Candidates should be able (in words or by using symbolic, graphical and numerical forms of presentation) to:
1. locate, select, organise and present information from a variety of sources
2. handle information, distinguishing the relevant from the extraneous
3. manipulate numerical and other data and translate information from one form to another
4. analyse and evaluate information so as to identify patterns, report trends and conclusions, and draw inferences
5. present reasoned explanations for phenomena, patterns and relationships
6. apply knowledge, including principles, to novel situations
7. bring together knowledge, principles, concepts and skills from different areas of chemistry, and apply them in a particular context
8. evaluate information and hypotheses
9. construct arguments to support hypotheses or to justify a course of action
10. demonstrate an awareness of the limitations of Chemistry theories and models.
These Assessment Objectives cannot be precisely specified in the syllabus content because questions testing such skills may be based on information which is unfamiliar to the candidate. In answering such questions, candidates are required to use principles and concepts that are within the syllabus and apply them in a logical, reasoned or deductive manner to a novel situation. Questions testing these objectives will often begin with one of the following words: predict, suggest, construct, calculate or determine (see the Glossary of Terms).
SCHEME OF ASSESSMENT
All candidates are required to enter for Papers 1 and 2.
Paper | Type of Paper | Duration | Weighting (%) | Marks |
1 | Multiple Choice | 1 h | 33 | 30 |
2 | Structured Questions | 2 h | 67 | 80 |
Paper 1 (1 h, 30 marks)
This paper consists of 30 compulsory multiple choice questions. Four to six items will be of the multiple completion type.
All questions will include 4 options.
Paper 2 (2 h, 80 marks)
This paper consists of two sections. All answers will be written in spaces provided on the Question Paper.
Section A (60 marks)
A variable number of structured questions including data-based questions, all compulsory. The data-based question(s) constitute(s) 15–20 marks for this paper. The data-based question(s) provide(s) a good opportunity to test higher order thinking skills such as handling, applying, and evaluating information.
Section B (20 marks)
Candidates will be required to answer one out of two questions. Each question will carry 20 marks.
These questions will require candidates to integrate knowledge and understanding from different areas and topics of the chemistry syllabus.
Weighting of Assessment Objectives
Assessment Objectives | Weighting (%) | Assessment Components | |
A | Knowledge with understanding | 40 | Papers 1, 2 |
B | Handling, applying and evaluating information | 60 | Papers 1, 2 |
ADDITIONAL INFORMATION
Data Booklet
A Data Booklet is available for use in the theory papers. The booklet is reprinted at the end of this syllabus document.
Nomenclature
Candidates will be expected to be familiar with the nomenclature used in the syllabus. The proposals in "Signs, Symbols and Systematics" (The Association for Science Education Companion to 16–19 Science, 2000) will generally be adopted although the traditional names sulfate, sulfite, nitrate, nitrite, sulfurous and nitrous acids will be used in question papers. Sulfur (and all compounds of sulfur) will be spelt with f (not with ph) in question papers, however candidates can use either spelling in their answers.
Units and significant figures
Candidates should be aware that misuse of units and/or significant figures, i.e. failure to quote units where necessary, the inclusion of units in quantities defined as ratios or quoting answers to an inappropriate number of significant figures, is liable to be penalised.
Disallowed Subject Combinations
Candidates may not simultaneously offer Chemistry at H1 and H2 levels.
CONTENT MAP
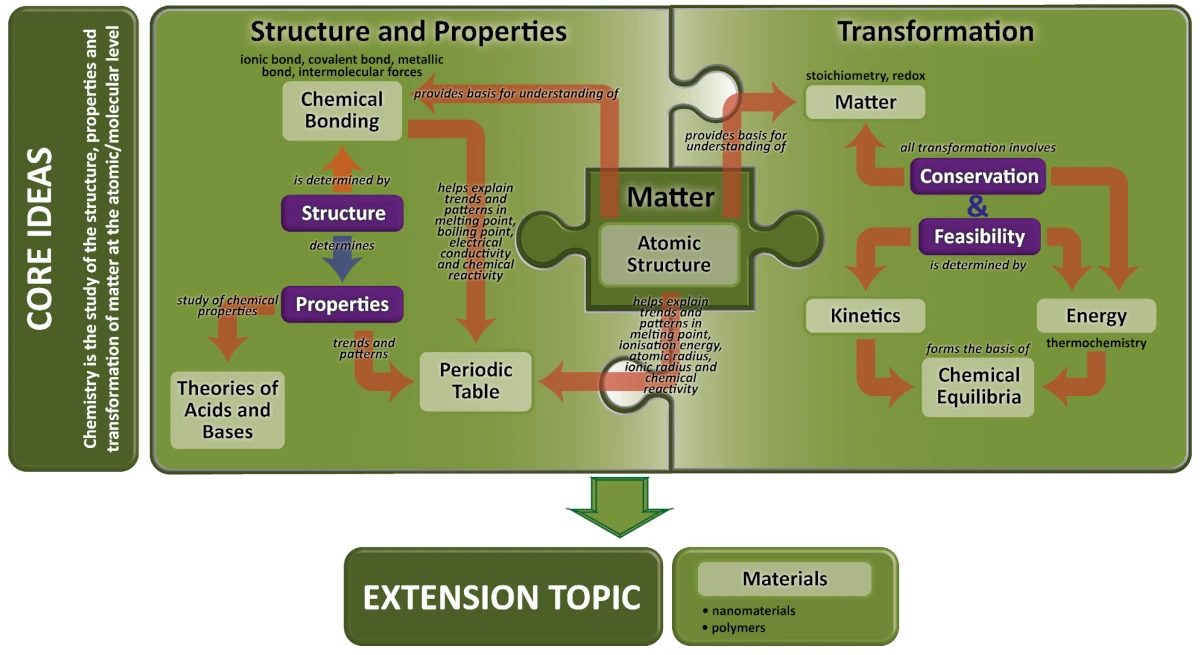
Chemistry is about the study of matter, its interactions and transformations. At a macroscopic level, we observe matter and its interactions everywhere in our daily life. The microscopic level looks at the structure of matter that gives rise to these interactions. At O-Level, students have been introduced to the fundamental idea that matter is made up of particles and the simple atomic model (electrons in discrete shells around a positively charged nucleus). This allows students to apply the key ideas of conservation of matter and energy in the quantitative treatment of reactions such as stoichiometry and thermochemistry.
At A-Level, an in-depth study of the electronic structure of atoms provides the basis for the study of chemical bonding. The Valence Shell Electron Pair Repulsion (VSEPR) model is used to visualise the three-dimensional structure of molecules, which determines the type of interactions possible and also helps to explain the physical and chemical properties. Knowledge of structure and bonding is also important to study and predict trends in properties of matter and its reactions.
Transformation of matter involves the study of the feasibility (energetics and kinetics considerations) and extent of chemical reactions (concept of equilibrium). The energetics dimension builds upon prior knowledge of thermochemistry, mainly enthalpy changes ( ΔH ). The chemical kinetics facet of a reaction can be understood quantitatively by relating the rate of reaction to concentration of reactants. The qualitative aspect which deals with the factors affecting rate of reactions will be covered based on the collision theory.
The concepts in chemical energetics and kinetics will form the basis for the study of Chemical Equilibrium. Theoretically all reactions are reversible, and the notion of dynamic equilibrium will be introduced. The concept of equilibrium constant ( K ) is understood via the equilibrium expression, which gives a measure of the extent of a reversible reaction. Factors which determine the position of equilibrium will also be examined. Chemical equilibria in aqueous media involving acids and bases will be dealt with in greater depth, in view of the relevance and prevalence of these concepts in real world contexts.
The extension topic on materials features applications of core concepts to a real-world context where specific examples, such as graphene and plastics, provide the opportunity for students to apply their knowledge on structure and bonding to understand the properties and uses of these materials.
This curriculum framework provides students the opportunity to appreciate the connections between the concepts in the Core Ideas of Matter, Structure and Properties, and Transformation, and to apply these to the study of materials in the Extension Topic.
SUBJECT CONTENT
CORE IDEA 1 – MATTER
1. Atomic Structure
Content
- The nucleus of the atom: neutrons and protons, isotopes, proton and nucleon numbers
- Electrons: electronic energy levels, ionisation energies, atomic orbitals, extranuclear structure
Learning Outcomes
Candidates should be able to:
(a) identify and describe protons, neutrons and electrons in terms of their relative charges and relative masses
(b) deduce the behaviour of beams of protons, neutrons and electrons in an electric field
(c) describe the distribution of mass and charges within an atom
(d) deduce the numbers of protons, neutrons and electrons present in both atoms and ions given proton and nucleon numbers (and charge)
(e) (i) describe the contribution of protons and neutrons to atomic nuclei in terms of proton number and nucleon number
(ii) distinguish between isotopes on the basis of different numbers of neutrons present
(f) describe the number and relative energies of the s, p and d orbitals for the principal quantum numbers 1, 2 and 3 and also the 4s and 4p orbitals
(g) describe the shapes of s and p orbitals
(h) state the electronic configuration of atoms and ions given the proton number (and charge)
(i) explain the factors influencing the ionisation energies of elements (see the Data Booklet) (see also Section 4)
(j) deduce the electronic configurations of elements from successive ionisation energy data
(k) interpret successive ionisation energy data of an element in terms of the position of that element within the Periodic Table
CORE IDEA 2 – STRUCTURE AND PROPERTIES
2. Chemical Bonding
Content
- Ionic bonding, metallic bonding, covalent bonding and co-ordinate (dative covalent) bonding
- Shapes of simple molecules and bond angles
- Bond polarities and polarity of molecules
- Intermolecular forces, including hydrogen bonding
- Bond energies and bond lengths
- Lattice structure of solids
- Bonding and physical properties
Learning Outcomes
Candidates should be able to:
(a) show understanding that all chemical bonds are electrostatic in nature and describe:
(i) ionic bond as the electrostatic attraction between oppositely charged ions
(ii) covalent bond as the electrostatic attraction between a shared pair of electrons and positively charged nuclei
(iii) metallic bond as the electrostatic attraction between a lattice of positive ions and delocalised electrons
(b) describe, including the use of ‘dot-and-cross’ diagrams,
(i) ionic bonding as in sodium chloride and magnesium oxide
(ii) covalent bonding as in hydrogen; oxygen; nitrogen; chlorine; hydrogen chloride; carbon dioxide; methane; ethene
(iii) co-ordinate (dative covalent) bonding, as in formation of the ammonium ion and in the Al2Cl6 molecule
(c) describe covalent bonding in terms of orbital overlap (limited to s and p orbitals only), giving σand π bonds (see also Section 9.2)
(d) explain the shapes of, and bond angles in, molecules such as BF3 (trigonal planar); CO2 (linear); CH4 (tetrahedral); NH3 (trigonal pyramidal); H2O (bent); SF6 (octahedral) by using the Valence Shell Electron Pair Repulsion theory
(e) predict the shapes of, and bond angles in, molecules analogous to those specified in (d)
(f) explain and deduce bond polarity using the concept of electronegativity
[quantitative treatment of electronegativity is not required]
(g) deduce the polarity of a molecule using bond polarity and its molecular shape (analogous to those specified in (d));
(h) describe the following forces of attraction (electrostatic in nature):
(i) intermolecular forces, based on permanent and induced dipoles, as in liquid and gaseous CHCl3(l); Br2(l) and the noble gases
(ii) hydrogen bonding, using ammonia and water as examples of molecules containing –NH and –OH groups
(i) outline the importance of intermolecular forces to the liquefaction of gases when subjected to high pressure and/or low temperature
(j) outline the importance of hydrogen bonding to the physical properties of substances, including ice and water
(k) explain the terms bond energy and bond length for covalent bonds
(l) compare the reactivities of covalent bonds in terms of bond energy, bond length and bond polarity
(m) describe, in simple terms, the lattice structure of a crystalline solid which is:
(i) ionic, as in sodium chloride and magnesium oxide
(ii) simple molecular, as in iodine
(iii) giant molecular, as in graphite and diamond
(iv) hydrogen-bonded, as in ice
(v) metallic, as in copper
[the concept of the ‘unit cell’ is not required]
(n) describe, interpret and/or predict the effect of different types of structure and bonding on the physical properties of substances
(o) suggest the type of structure and bonding present in a substance from given information
3. Theories of Acids and Bases
Content
- Arrhenius and Brønsted-Lowry theories of acids and bases
- Acid dissociation constants, Ka
- Base dissociation constants, Kb
- The ionic product of water, Kw
- pH: choice of indicators
- Buffer solutions
Learning Outcomes
Candidates should be able to:
(a) show understanding of, and apply the Arrhenius theory of acids and bases
(b) show understanding of, and apply the Brønsted-Lowry theory of acids and bases, including the concept of conjugate acids and conjugate bases
(c) explain qualitatively the differences in behaviour between strong and weak acids and bases in terms of the extent of dissociation
(d) explain the terms pH; Ka; Kb; Kw
[the relationship Kw = KaKb is not required]
(e) calculate [H+(aq)] and pH values for strong acids and strong bases
(f) explain the choice of suitable indicators for acid-base titrations, given appropriate data, in terms of the strengths of the acids and bases
(g) (i) explain how buffer solutions control pH
(ii) describe and explain their uses including the role of H2CO3/HCO3– in controlling pH in blood
4. The Periodic Table
Content
- Periodicity of atomic and physical properties of the elements: variation with proton number across the third period (sodium to chlorine) and down Group 17 of:
(i) electronic configuration
(ii) atomic radius and ionic radius
(iii) ionisation energy
(iv) electronegativity
(v) melting point
(vi) electrical conductivity
- Periodicity of chemical properties of the elements in the third period:
(i) variation in oxidation number and bonding of the oxides (sodium to sulfur only) and of the chlorides (sodium to phosphorus only)
(ii) reactions of these oxides and chlorides with water
(iii) acid/base behaviour of these oxides and the corresponding hydroxides
- Periodicity of chemical properties of the elements down the group (Group 1 and Group 17):
(i) as reducing agents (Group 1) and oxidising agents (Group 17)
(ii) thermal stability of Group 17 hydrides
Learning Outcomes
Trends and variations in atomic and physical properties
For elements in the third period (sodium to chlorine), and in Group 17 (chlorine to iodine) ,candidates should be able to:
(a) recognise variation in the electronic configurations across a Period and down a Group
(b) describe and explain qualitatively the general trends and variations in atomic radius, ionic radius, first ionisation energy and electronegativity:
(i) across a Period in terms of shielding and nuclear charge
(ii) down a Group in terms of increasing number of electronic shells, shielding and nuclear charge
(c) interpret the variation in melting point and in electrical conductivity across a Period in terms of structure and bonding in the elements (metallic, giant molecular, or simple molecular)
(d) describe and explain the trend in volatility of the Group 17 elements in terms of instantaneous dipole- induced dipole attraction
Trends and variations in chemical properties
For elements in the third period (sodium to chlorine) candidates should be able to:
(e) (i) state and explain the variation in the highest oxidation number of the elements in oxides (for Na2O; MgO; Al2O3; SiO2; P4O10; SO3) and chlorides (for NaCl; MgCl2; AlCl3; SiCl4; PCl5)
(ii) state and explain the variation in bonding in oxides and chlorides in terms of electronegativity (with the exception of AlCl3)
(iii) describe the reactions of the oxides with water (for Na2O; MgO; Al2O3; SiO2; P4O10; SO3)
(iv) describe and explain the acid/base behaviour of oxides (for Na2O; MgO; Al2O3; SiO2; P4O10; SO3) and hydroxides (for NaOH; Mg(OH)2; Al(OH)3), including, where relevant, amphoteric behaviour in reaction with sodium hydroxide (only) and acids
(v) describe and explain the reactions of the chlorides with water (for NaCl; MgCl2; AlCl3; SiCl4; PCl5)
(vi) suggest the types of structure and bonding present in the oxides and chlorides from observations of their chemical and physical properties
For elements in Group 1 (lithium to caesium) and Group 17 (chlorine to iodine) candidates should be able to:
(f) describe and explain the relative reactivity of elements of:
(i) Group 1 as reducing agents in terms of ease of loss of electrons
(ii) Group 17 as oxidising agents in terms of ease of gain of electrons
(g) describe and explain the trend in thermal stability of Group 17 hydrides in terms of bond energies
In addition, candidates should be able to:
(h) predict the characteristic properties of an element in a given Group by using knowledge of chemical periodicity
(i) deduce the nature, possible position in the Periodic Table, and identity of unknown elements from given information of physical and chemical properties
CORE IDEA 3 – TRANSFORMATION
5. The Mole Concept and Stoichiometry
Content
- Relative masses of atoms and molecules
- The mole, the Avogadro constant
- The calculation of empirical and molecular formulae
- Reacting masses and volumes (of solutions and gases)
- Redox processes: electron transfer and changes in oxidation number (oxidation state)
Learning Outcomes
[the term relative formula mass or Mr will be used for ionic compounds]
Candidates should be able to:
(a) define the terms relative atomic, isotopic, molecular and formula mass
(b) define the term mole in terms of the Avogadro constant
(c) calculate the relative atomic mass of an element given the relative abundances of its isotopes
(d) define the terms empirical and molecular formula
(e) calculate empirical and molecular formulae using combustion data or composition by mass
(f) write and/or construct balanced equations
(g) describe and explain redox processes in terms of electron transfer and/or of changes in oxidation number (oxidation state) as exemplified by Fe3+/Fe2+ and MnO4– /Mn2+
(h) construct redox equations using the relevant half-equations
(i) perform calculations, including use of the mole concept, involving:
(i) reacting masses (from formulae and equations)
(ii) volumes of gases (e.g. in the burning of hydrocarbons)
(iii) volumes and concentrations of solutions
[when performing calculations, candidates’ answers should reflect the number of significant figures given or asked for in the question]
(j) deduce stoichiometric relationships from calculations such as those in (i)
6. Chemical Energetics: Thermochemistry
Content
- Enthalpy changes: ΔH, of formation; combustion; neutralisation; bond energy; lattice energy
- Hess’ Law
Learning Outcomes
Candidates should be able to:
(a) explain that most chemical reactions are accompanied by energy changes, principally in the form of heat usually associated with the breaking and forming of chemical bonds; the reaction can be exothermic (ΔH negative) or endothermic (ΔH positive)
(b) construct and interpret an energy profile diagram, in terms of the enthalpy change of the reaction and of the activation energy (see also Section 7)
(c) explain and use the terms:
(i) enthalpy change of reaction and standard conditions, with particular reference to: formation; combustion; neutralisation
(ii) bond energy (ΔH positive, i.e. bond breaking) (see also Section 2)
(iii) lattice energy (ΔH negative, i.e. gaseous ions to solid lattice)
(d) calculate enthalpy changes from appropriate experimental results, including the use of the relationship: heat change = mcΔT
(e) explain, in qualitative terms, the effect of ionic charge and of ionic radius on the numerical magnitude of a lattice energy
(f) apply Hess’ Law to carry out calculations involving given simple energy cycles and relevant energy terms (restricted to enthalpy changes of formation, combustion and neutralisation), with particular reference to:
(i) determining enthalpy changes that cannot be found by direct experiment, e.g. an enthalpy change of formation from enthalpy changes of combustion
(ii) average bond energies
[construction of energy cycles is not required]
7. Reaction Kinetics
Content
- Simple rate equations; orders of reaction; rate constants
- Concept of activation energy
- Effect of concentration, temperature, and catalysts on reaction rate
- Heterogeneous catalysts
- Enzymes as biological catalysts
Learning Outcomes
Candidates should be able to:
(a) explain and use the terms: rate of reaction; rate equation; order of reaction; rate constant; half-life of a reaction; activation energy; catalysis
(b) construct and use rate equations of the form rate = k[A]m[B]n (limited to simple cases of single-step reactions and of multi-step processes with a rate-determining step, for which m and n are 0, 1 or 2), including:
(i) deducing the order of a reaction by the initial rates method
(ii) justifying, for zero- and first-order reactions, the order of reaction from concentration-time graphs
(iii) calculating an initial rate using concentration data
[integrated forms of rate equations are not required]
(c) show understanding that the half-life of a first-order reaction is independent of concentration
(d) explain qualitatively, in terms of frequency of collisions, the effect of concentration changes on the rate of a reaction
(e) show understanding, including reference to the Boltzmann distribution, of what is meant by the term activation energy
(f) explain qualitatively, in terms both of the Boltzmann distribution and of collision frequency, the effect of temperature change on a rate constant (and hence, on the rate) of a reaction
(g) (i) explain that, in the presence of a catalyst, a reaction follows a different pathway, i.e. one of lower activation energy, giving a larger rate constant
(ii) interpret this catalytic effect in terms of the Boltzmann distribution
(h) outline the mode of action of heterogeneous catalysis, as exemplified by the catalytic removal of oxides of nitrogen in the exhaust gases from car engines (see also Section 9.1)
(i) describe enzymes as biological catalysts which may have specific activity
8. Chemical Equilibria
Content
- Chemical equilibria: reversible reactions; dynamic equilibrium
(i) factors affecting chemical equilibria
(ii) equilibrium constants
(iii) the Haber process
Learning Outcomes
Candidates should be able to:
(a) explain, in terms of rates of the forward and reverse reactions, what is meant by a reversible reaction and dynamic equilibrium
(b) state Le Chatelier’s Principle and apply it to deduce qualitatively (from appropriate information) the effects of changes in concentration, pressure or temperature, on a system at equilibrium
(c) deduce whether changes in concentration, pressure or temperature or the presence of a catalyst affect the value of the equilibrium constant for a reaction
(d) deduce expressions for equilibrium constants in terms of concentrations, Kc
(e) calculate the values of equilibrium constants in terms of concentrations from appropriate data
(f) calculate the quantities present at equilibrium, given appropriate data (such calculations will not require the solving of quadratic equations)
(g) describe and explain the conditions used in the Haber process, as an example of the importance of an understanding of chemical equilibrium in the chemical industry
EXTENSION TOPICS - MATERIALS
9.1 Nanomaterials
Content
- Nanomaterials and nanoparticles
- Heterogenous catalysis
- Structure and properties of graphene
Learning Outcomes
Candidates should be able to:
(a) define the terms nanomaterials and nanoparticles
(b) describe the large surface area to volume ratio of nanomaterials, explaining the effects on the following:
(i) catalysis as exemplified by the use of platinum nanoparticles in catalytic converters (see also Section 7)
(ii) interactions as exemplified by the wall climbing ability of geckos
(c) describe the structure of graphene, a nanomaterial, and relate the following properties to its structure:
(i) electrical conductivity
(ii) tensile strength
(d) recognise the potential effect of nanoparticles on human health and the environment
9.2 Polymers
Preamble
Although there are features of organic chemistry topics that are distinctive, it is intended that appropriate cross-references with other sections/topics in the syllabus should be made.
When describing preparative reactions, candidates will be expected to quote the reagents, e.g. aqueous NaOH, the essential practical conditions, e.g. reflux, high temperature and pressure, and the identity of each of the major products. Detailed conditions involving specific temperature and pressure values are not required.
Detailed knowledge of practical procedures is also not required: however, candidates may be expected to suggest (from their knowledge of the reagents, essential conditions and products) what steps may be needed to purify/extract a required product from the reaction mixture. In equations for organic redox reactions, the symbols [O] and [H] are acceptable.
Candidates will be expected to be able to predict the reaction products of a given compound in reactions that are chemically similar to those specified in the syllabus.
Content
- Empirical, molecular and structural formulae
- Functional groups and the naming of organic compounds
- Common terms for organic reactions
- Isomerism: constitutional (structural); cis-trans
- Shapes of organic molecules; σ and π bonds
- Alkanes (as exemplified by ethane)
(i) combustion and substitution reaction
- Alkenes (as exemplified by ethene)
(i) combustion and addition reactions
- Halogenoalkanes (as exemplified by bromoethane)
(i) substitution
(ii) elimination
- Aldehydes (as exemplified by ethanal)
(i) oxidation to carboxylic acid
(ii) reduction
- Ketones (as exemplified by propanone)
(i) reduction
- Alcohols (as exemplified by ethanol)
(i) combustion
(ii) oxidation to carboxylic acid
(iii) elimination
- Carboxylic acids (as exemplified by ethanoic acid)
(i) ester formation
(ii) amide formation
- Structure and uses of polymers
Candidates are expected to be able to interpret and use the following types of representations in the description of organic molecules. The examples given are for the compound lactic acid.
Empirical Formula: simplest ratio of the number of atoms of the elements present in one molecule, e.g. CH2O
Molecular Formula: actual number of atoms of the elements present in one molecule, e.g. C3H6O3
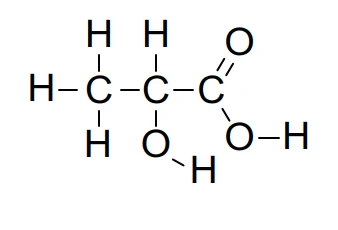
Structural Formula: shows how the constituent atoms of a molecule are joined together with minimal detail, using conventional groups, for an unambiguous structure, e.g. CH3CH(OH)CO2H
Full Structural or Displayed Formula: detailed structure of molecule showing the relative placing of atoms and the number of bonds between them, e.g.
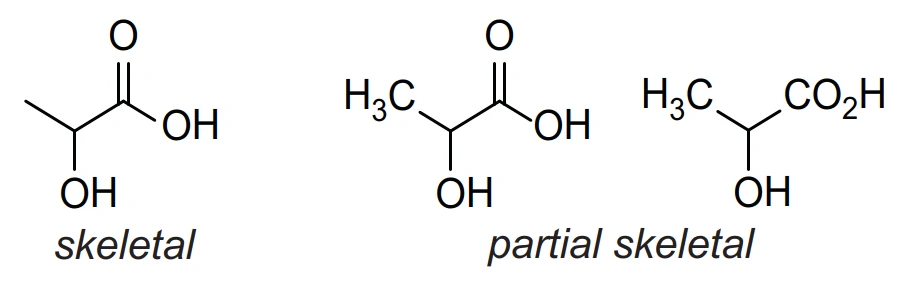
Skeletal Formula: simplified representation of an organic formula derived from the structural formula by removing hydrogen atoms (and their associated bonds) and carbon atoms from alkyl chains, leaving just the carbon-carbon bonds in the carbon skeleton and the associated functional groups
Skeletal or partial skeletal representations may be used in question papers and are acceptable in candidates’ answers where they are unambiguous, e.g.
The following convention for representing the aromatic ring is preferred:
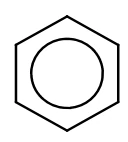
Learning Outcomes
Candidates should be able to:
(a) interpret, and use the nomenclature, general formulae and structural formulae (including displayed formulae) of the following classes of compounds:
(i) hydrocarbons (alkanes, alkenes and benzene)
(ii) halogenoalkanes
(iii) alcohols (including primary, secondary and tertiary)
(iv) aldehydes and ketones
(v) carboxylic acids
(vi) esters
(vii) amines
(viii) amides
(b) interpret, and use the following terminology associated with organic reactions:
(i) functional group
(ii) addition, substitution, elimination
(iii) condensation, hydrolysis
(iv) oxidation and reduction
[in equations for organic redox reactions, the symbols [O] and [H] are acceptable]
(c) describe constitutional (structural) isomerism
(d) describe cis-trans isomerism in alkenes, and explain its origin in terms of restricted rotation due to the presence of π bonds
[use of E , Z nomenclature is not required]
(e) deduce the possible isomers for an organic molecule of known molecular formula
(f) (i) describe the shapes of the ethane, ethene and benzene molecules
(ii) explain the shapes of, and bond angles, in the ethane, ethene and benzene molecules in relation to σ and π carbon-carbon bonds
[knowledge of hybridisation is not required]
(iii) predict the shapes of, and bond angles in, molecules analogous to those specified in (f)(ii)
(g) describe the chemistry of the following classes of compounds:
(i) alkanes (exemplified by ethane) as being generally unreactive except in terms of combustion and substitution by chlorine
(ii) alkenes (exemplified by ethene) in terms of combustion and addition reactions with bromine (in CCl4) and hydrogen
(iii) halogenoalkanes (exemplified by bromoethane) in terms of substitution reaction to alcohols and elimination reactions to alkenes
(iv) aldehydes (exemplified by ethanal) and ketones (exemplified by propanone) in terms of their reduction to primary and secondary alcohols respectively; and oxidation of aldehydes to carboxylic acids
(v) alcohols (exemplified by ethanol) in terms of combustion, oxidation to carboxylic acids and elimination to alkenes
(vi) carboxylic acids (exemplified by ethanoic acid) in terms of condensation with alcohols to form esters (in the presence of concentrated sulfuric acid), and with amines (exemplified by ethylamine) to form amides (in the presence of dicyclohexylcarbodiimide, DCC)
[knowledge of structure of DCC is not required]
(vii) esters (exemplified by ethyl ethanoate) and amides (exemplified by ethanamide) in terms of hydrolysis with acids and bases
[detailed conditions involving specific temperature and pressure values are not required]
(h) recognise polymers as macromolecules built up from monomers, with average molar mass of at least 1000 or at least 100 repeat units
(i) classify and explain the difference between addition and condensation polymers
(j) classify and explain the difference between thermoplastic (linear, as exemplified by poly(ethene)) and thermosetting (cross-linked, as exemplified by poly(diallyl phthalate)) polymers with reference to structure, bonding and the following properties:
(i) softening behaviour, including capacity to be recycled
(ii) rigidity
(iii) strength
(k) describe and explain the types of structure and bonding in relation to the properties and uses as exemplified by the following:
(i) low density poly(ethene) (LDPE) in plastic bag and high density poly(ethene) (HDPE) in plastic bottles in relation to LDPE being softer and more flexible, and HDPE being harder and stiffer
(ii) polyester and polyamide as fabric in relation to polyester (exemplified by poly(ethylene terephthalate) (PET)) as a fabric that is slightly less prone to creasing than polyamide (exemplified by nylon 6,6)
(iii) poly(vinyl alcohol) (PVA) as a water-soluble polymer in eye drops and poly(vinyl chloride) (PVC) as a water-resistant polymer used in raincoats
(iv) poly(propene) (PP) container instead of one made from poly(ethylene terephthalate) (PET) to store strongly alkaline cleaning solutions due to hydrolysis of PET
(l) predict physical properties of polymer from its structure
(m) recognise that materials are a finite resource and the importance of recycling plastics, considering the economic, environmental and social factors
SUMMARY OF KEY QUANTITIES AND UNITS
The list below is intended as a guide to the more important quantities which might be encountered in teaching and used in question papers. The list is not exhaustive.
Quantity | Usual symbols | Unit |
Base quantities | ||
amount of substance | n | mol |
electric current | I | A |
length | l | m |
mass | m | kg, g |
thermodynamic temperature | T | K |
time | t | s |
Other quantities | ||
acid dissociation constant | Ka | mol dm–3 |
atomic mass | ma | g, kg |
Avogadro constant | L | mol–1 |
base dissociation constant | Kb | mol dm–3 |
bond energy | – | kJ mol–1 |
charge on the electron | e | C |
concentration | c | mol dm–3 |
density | ρ | kg m–3, g dm–3, g cm–3 |
electric potential difference | V | V |
electromotive force | E | V |
electron affinity | – | kJ mol–1 |
enthalpy change of reaction | ΔH | J, kJ |
equilibrium constant | K, Kp, Kc | as appropriate |
Faraday constant | F | C mol–1 |
frequency | v, f | Hz |
half-life | T½, t½ | s |
ionic product, solubility product | K, Ksp, | as appropriate |
ionic product of water | Kw | mol2 dm–6 |
ionisation energy | I | kJ mol–1 |
lattice energy | – | kJ mol–1 |
molar gas constant | R | J K–1 mol–1 |
molar mass | M | g mol–1 |
mole fraction | x | – |
molecular mass | m | g, kg |
neutron number | N | – |
nucleon number | A | – |
number of molecules | N | – |
number of molecules per unit volume | n | m–3 |
order of reaction | n, m | – |
partition coefficient | K | – |
Planck constant | h | J s |
pH | pH | – |
pressure | p | Pa |
proton number | Z | – |
rate constant | k | as appropriate |
relative atomic or isotopic mass | Ar | – |
relative molecular mass | Mr | – |
speed of electromagnetic waves | c | m s–1 |
(standard) electrode or redox potential | (E)θ E | V |
standard enthalpy change of reaction | ΔHθ | J mol–1, kJ mol–1 |
temperature | θ, t | °C |
volume | V, v | m3, dm3 |
wavelength | λ | m, mm, nm |
MATHEMATICAL REQUIREMENTS
It is assumed that candidates will be competent in the techniques described below.
Make calculations involving addition, subtraction, multiplication and division of quantities. Make approximate evaluations of numerical expressions.
Express small fractions as percentages, and vice versa.
Calculate an arithmetic mean.
Transform decimal notation to power of ten notation (standard form).
Use calculators to evaluate logarithms, squares, square roots, and reciprocals.
Change the subject of an equation. (Most such equations involve only the simpler operations but may include positive and negative indices and square roots.)
Substitute physical quantities into an equation using consistent units so as to calculate one quantity. Check the dimensional consistency of such calculations, e.g. the units of a rate constant k.
Solve simple algebraic equations.
Comprehend and use the symbols/notations <, >, ≈, /, Δ, ≡, x̄ (or <x>).
Test tabulated pairs of values for direct proportionality by a graphical method or by constancy of ratio.
Select appropriate variables and scales for plotting a graph, especially to obtain a linear graph of the form y = mx + c.
Determine and interpret the slope and intercept of a linear graph.
Choose by inspection a straight line that will serve as the ‘least bad’ linear model for a set of data presented graphically.
Understand (i) the slope of a tangent to a curve as a measure of rate of change, (ii) the ‘area’ below a curve where the area has physical significance, e.g. Boltzmann distribution curves.
Comprehend how to handle numerical work so that significant figures are neither lost unnecessarily nor used beyond what is justified.
Estimate orders of magnitude.
Formulate simple algebraic equations as mathematical models, e.g. construct a rate equation, and identify failures of such models.
Calculators
Any calculator used must be on the Singapore Examinations and Assessment Board list of approved calculators.
GLOSSARY OF TERMS
It is hoped that the glossary (which is relevant only to science subjects) will prove helpful to candidates as a guide, i.e. it is neither exhaustive nor definitive. The glossary has been deliberately kept brief not only with respect to the number of terms included but also to the descriptions of their meanings. Candidates should appreciate that the meaning of a term must depend in part on its context.
1. Define (the term(s)...) is intended literally, only a formal statement or equivalent paraphrase being required.
2. What do you understand by/What is meant by (the term(s)...) normally implies that a definition should be given, together with some relevant comment on the significance or context of the term(s) concerned, especially where two or more terms are included in the question. The amount of supplementary comment intended should be interpreted in the light of the indicated mark value.
3. State implies a concise answer with little or no supporting argument, e.g. a numerical answer that can be obtained ‘by inspection’.
4. List requires a number of points, generally each of one word, with no elaboration. Where a given number of points is specified, this should not be exceeded.
5. Explain may imply reasoning or some reference to theory, depending on the context.
6. Describe requires candidates to state in words (using diagrams where appropriate) the main points of the topic. It is often used with reference either to particular phenomena or to particular experiments. In the former instance, the term usually implies that the answer should include reference to (visual) observations associated with the phenomena.
In other contexts, describe and give an account of should be interpreted more generally, i.e. the candidate has greater discretion about the nature and the organisation of the material to be included in the answer. Describe and explain may be coupled in a similar way to state and explain.
7. Discuss requires candidates to give a critical account of the points involved in the topic.
8. Outline implies brevity, i.e. restricting the answer to giving essentials.
9. Predict implies that the candidate is not expected to produce the required answer by recall but by making a logical connection between other pieces of information. Such information may be wholly given in the question or may depend on answers extracted in an early part of the question.
10. Deduce is used in a similar way as predict except that some supporting statement is required, e.g. reference to a law/principle, or the necessary reasoning is to be included in the answer.
11. Comment is intended as an open-ended instruction, inviting candidates to recall or infer points of interest relevant to the context of the question, taking account of the number of marks available.
12. Suggest is used in two main contexts, i.e. either to imply that there is no unique answer (e.g. in chemistry, two or more substances may satisfy the given conditions describing an ‘unknown’), or to imply that candidates are expected to apply their general knowledge to a ‘novel’ situation, one that may be formally ‘not in the syllabus’.
13. Find is a general term that may variously be interpreted as calculate, measure, determine, etc.
14. Calculate is used when a numerical answer is required. In general, working should be shown, especially where two or more steps are involved.
15. Measure implies that the quantity concerned can be directly obtained from a suitable measuring instrument, e.g. length, using a rule, or angle, using a protractor.
16. Determine often implies that the quantity concerned cannot be measured directly but is obtained by calculation, substituting measured or known values of other quantities into a standard formula, e.g. relative molecular mass.
17. Estimate implies a reasoned order of magnitude statement or calculation of the quantity concerned, making such simplifying assumptions as may be necessary about points of principle and about the values of quantities not otherwise included in the question.
18. Sketch, when applied to graph work, implies that the shape and/or position of the curve need only be qualitatively correct, but candidates should be aware that, depending on the context, some quantitative aspects may be looked for, e.g. passing through the origin, having an intercept, asymptote or discontinuity at a particular value.
In diagrams, sketch implies that a simple, freehand drawing is acceptable: nevertheless, care should be taken over proportions and the clear exposition of important details.
19. Construct is often used in relation to chemical equations where a candidate is expected to write a balanced equation, not by factual recall but by analogy or by using information in the question.
20. Compare requires candidates to provide both the similarities and differences between things or concepts.
21. Classify requires candidates to group things based on common characteristics.\
22. Recognise is often used to identify facts, characteristics or concepts that are critical (relevant/appropriate) to the understanding of a situation, event, process or phenomenon.
TEXTBOOKS AND REFERENCES
Teachers may find reference to the following books helpful.
Cambridge International AS and A Level Chemistry by Peter Cann and Peter Hughes, published by Hodder Education
Cambridge International AS and A Level Chemistry Coursebook with CD-ROM (2nd Edition) by Lawrie Ryan and Roger Norris, published by Cambridge University Press
A-Level Chemistry (4th Edition) by E N Ramsden, published by Oxford University Press
Understanding Chemistry for Advanced Level (3rd Edition), by Ted Lister and Janet Renshaw, published by Oxford University Press
AS and A Level Chemistry through Diagrams by Michael Lewis, published by Oxford University Press
Chemistry in Context (6th Edition) by Graham Hill and John Holman, published by Oxford University Press
Chemistry in Context Laboratory Manual and Study Guide (5th Edition) by Graham Hill and John Holman, published by Oxford University Press
Experiments and Exercises in Basic Chemistry (7th Edition) by Steve Murov and Brian Stedjee, published by Wiley
Chemical Ideas (Salters Advanced Chemistry) by Adelene Cogill, et al., published by Pearson Education Limited
Science at the nanoscale: An introductory textbook by Chin Wee Shong, Sow Chorng Haur and Andrew T S Wee, published by Pan Stanford Publishing
The Language of Mathematics in Science: A Guide for Teachers of 11–16 Science (2016) by R Boohan, published by the Association for Science Education. ISBN 9780863574559.
https://www.ase.org.uk/mathsinscience
Teachers are encouraged to choose texts for class use which they feel will be of interest to their students and will support their own teaching style.
Many publishers are also producing videos and software appropriate for A-Level Chemistry students.
