Singapore GCE A Level H3 Chemistry Syllabus 9813
Disclaimer: This is the web version of Singapore–Cambridge GCE Advanced Level H3 Chemistry (Syllabus 9813) for viewers who prefer this to the pdf format, painstakingly reproduced by Chemistry Guru, Singapore's top JC Chemistry tuition centre. Minor adjustments are made for alignment and easier web navigation.
To access the original pdf file from SEAB website please click here.
CONTENTS
1. SPECTROSCOPIC TECHNIQUES
1.1 Basic Principles of Spectroscopy
1.2 Ultraviolet/visible Spectroscopy
1.3 Infra-red (IR) Spectroscopy
1.4 Nuclear Magnetic Resonance (NMR) Spectroscopy
2. FURTHER ORGANIC MECHANISMS
2.2 Basic Physical Organic Chemistry
SUMMARY OF KEY QUANTITIES AND UNITS
INTRODUCTION
The H3 Chemistry syllabus has been designed to build on and extend the knowledge, understanding and skills acquired from the H2 Chemistry syllabus. It caters to students of strong ability and keen interest in chemistry, and is designed with an emphasis on independent and self-directed learning. The H3 Chemistry syllabus provides greater depth and rigour in the subject for students pursuing further studies in chemistry-related fields.
Students should simultaneously offer H2 Chemistry, and will be assumed to have knowledge and understanding of chemistry at H2 level.
AIMS
The aims of a course based on this syllabus should be to:
1. provide students with an experience that deepens their knowledge and skills in chemistry, and foster attitudes necessary for further studies in related fields
2. develop in students the appreciation of the practice, value and rigour of chemistry as a discipline
3. develop in students the skills to analyse chemical issues, and to apply relevant concepts and techniques to solve problems.
PRACTICES OF SCIENCE
Science as a discipline is more than the acquisition of a body of knowledge (e.g. scientific facts, concepts, laws, and theories); it is a way of knowing and doing. It includes an understanding of the nature of scientific knowledge and how this knowledge is generated, established and communicated. Scientists rely on a set of established procedures and practices associated with scientific inquiry to gather evidence and test their ideas on how the natural world works. However, there is no single method and the real process of science is often complex and iterative, following many different paths. While science is powerful, generating knowledge that forms the basis for many technological feats and innovations, it has limitations.
The Practices of Science are explicitly articulated in this syllabus to allow teachers to embed them as learning objectives in their lessons. Students’ understanding of the nature and limitations of science and scientific inquiry are developed effectively when the practices are taught in the context of relevant science content. Attitudes relevant to science such as inquisitiveness, concern for accuracy and precision, objectivity, integrity and perseverance should be emphasised in the teaching of these practices where appropriate. For example, students learning science should be introduced to the use of technology as an aid in practical work or as a tool for the interpretation of experimental and theoretical results.
The Practices of Science comprise three components:
1. Understanding the Nature of Scientific Knowledge
1.1 Understand that science is an evidence-based, model-building enterprise concerned with the natural world
1.2 Understand that the use of both logic and creativity is required in the generation of scientific knowledge
1.3 Recognise that scientific knowledge is generated from consensus within the community of scientists through a process of critical debate and peer review
1.4 Understand that scientific knowledge is reliable and durable, yet subject to revision in the light of new evidence
2. Demonstrating Science Inquiry Skills
2.1 Identify scientific problems, observe phenomena and pose scientific questions/hypotheses
2.2 Plan and conduct investigations by selecting the appropriate experimental procedures, apparatus and materials, with due regard for accuracy, precision and safety
2.3 Obtain, organise and represent data in an appropriate manner
2.4 Analyse and interpret data
2.5 Construct explanations based on evidence and justify these explanations through reasoning and logical argument
2.6 Use appropriate models1 to explain concepts, solve problems and make predictions
2.7 Make decisions based on evaluation of evidence, processes, claims and conclusions
2.8 Communicate scientific findings and information using appropriate language and terminology
3. Relating Science and Society
3.1 Recognise that the application of scientific knowledge to problem solving could be influenced by other considerations such as economic, social, environmental and ethical factors
3.2 Demonstrate an understanding of the benefits and risks associated with the application of science to society
3.3 Use scientific principles and reasoning to understand, analyse and evaluate real-world systems as well as to generate solutions for problem solving
1 A model is a representation of an idea, an object, a process or a system that is used to describe and explain phenomena that cannot be experienced directly. Models exist in different forms, from the concrete, such as physical scale models, to the abstract, such as diagrams or mathematical expressions. The use of models involves the understanding that all models contain approximations and assumptions limiting their validity and predictive power.
CURRICULUM FRAMEWORK
The curriculum framework for H3 Chemistry builds on the framework for H2 Chemistry as illustrated in Fig. 1.
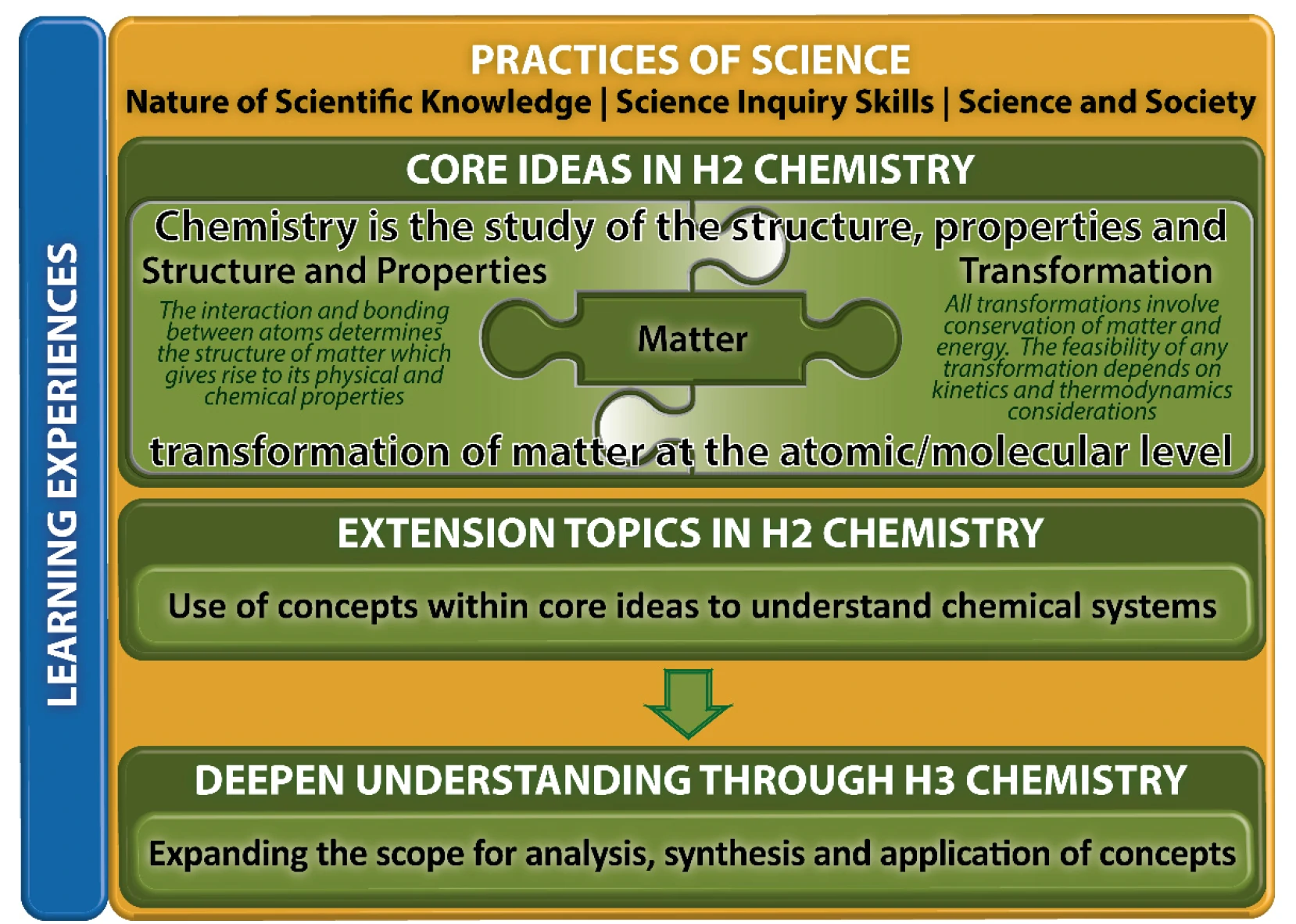
Fig. 1: H3 Chemistry Curriculum Framework
1. Content Topics
The content topics in the H3 Chemistry are organised into two levels underpinned by the Practices of Science:
(a) Core ideas and Extension topics in H2 Chemistry: These are elaborated in the corresponding H2 Chemistry syllabus.
(b) Additional content in H3 Chemistry: Two content areas Spectroscopic Techniques and Further Organic Mechanisms are included to deepen the understanding of the core ideas by expanding the scope for analysis, synthesis and application of concepts.
2. Practices of Science
The Practices of Science are common to the natural sciences of physics, chemistry and biology. These practices highlight the ways of thinking and doing that are inherent in the scientific approach, with the aim of equipping students with the understanding, skills, and attitudes shared by the scientific disciplines, including an appropriate approach to ethical issues.
3. Learning Experiences
The Learning Experiences2 refer to a range of learning opportunities selected by teachers to link the chemistry content with the Core Ideas and the Practices of Science to enhance students’ learning of the concepts. Rather than being mandatory, teachers are encouraged to incorporate Learning Experiences that match the interests and abilities of their students and provide opportunities to illustrate and exemplify the Practices of Science, where appropriate. Real-world contexts can help illustrate the concepts in chemistry and their applications. Experimental activities and ICT tools can also be used to build students’ understanding.
2 The Learning Experiences can be found in the Teaching and Learning syllabus.
ASSESSMENT OBJECTIVES
The Assessment Objectives listed below reflect those parts of the Aims and Practices of Science that will be assessed.
A. Knowledge with understanding
Candidates should be able to demonstrate knowledge and understanding in relation to:
1. scientific phenomena, facts, laws, definitions, concepts and theories
2. scientific vocabulary, terminology and conventions (including symbols, quantities and units)
3. scientific instruments and apparatus, including techniques of operation and aspects of safety
4. scientific quantities and their determination
5. scientific and technological applications with their social, economic and environmental implications.
The syllabus content defines the factual knowledge that candidates may be required to recall and explain. Questions testing these objectives will often begin with one of the following words: define, state, name, describe, explain or outline (see the Glossary of Terms).
B. Handling, applying and evaluating information
Candidates should be able (in words or by using symbolic, graphical and numerical forms of presentation) to:
1. locate, select, organise and present information from a variety of sources
2. handle information, distinguishing the relevant from the extraneous
3. manipulate numerical and other data and translate information from one form to another
4. analyse and evaluate information so as to identify patterns, report trends and conclusions, and draw inferences
5. present reasoned explanations for phenomena, patterns and relationships
6. apply knowledge, including principles, to novel situations
7. bring together knowledge, principles, concepts and skills from different areas of chemistry, and apply them in a particular context
8. evaluate information and hypotheses
9. construct arguments to support hypotheses or to justify a course of action
10. demonstrate an awareness of the limitations of Chemistry theories and models.
These Assessment Objectives cannot be precisely specified in the syllabus content because questions testing such skills may be based on information which is unfamiliar to the candidate. In answering such questions, candidates are required to use principles and concepts that are within the syllabus and apply them in a logical, reasoned or deductive manner to a novel situation. Questions testing these objectives will often begin with one of the following words: predict, suggest, construct, calculate or determine (see the Glossary of Terms).
SCHEME OF ASSESSMENT
Candidates will take a 2 h 30 min paper (100 marks total). This paper consists of two sections and will include questions that require candidates to integrate knowledge and understanding from different sections in the syllabus.
Section A (60 marks)
This section will consist of a variable number of compulsory free response questions including 1 or 2 stimulus-based questions. The stimulus-based question(s) constitute(s) 15–20 marks for this paper.
Section B (40 marks)
Candidates will be required to answer two out of three free response questions. Each question will carry 20 marks.
Weighting of Assessment Objectives
Assessment Objectives | Weighting (%) | |
A | Knowledge with understanding | 25 |
B | Handling, applying and evaluating information | 75 |
ADDITIONAL INFORMATION
Data Booklet
A Data Booklet is available for use in the theory papers. The booklet is reprinted at the end of this syllabus document.
Nomenclature
Candidates will be expected to be familiar with the nomenclature used in the syllabus. The proposals in "Signs, Symbols and Systematics" (The Association for Science Education Companion to 16–19 Science, 2000) will generally be adopted although the traditional names sulfate, sulfite, nitrate, nitrite, sulfurous and nitrous acids will be used in question papers. Sulfur (and all compounds of sulfur) will be spelt with f (not with ph) in question papers, however candidates can use either spelling in their answers.
Units and significant figures
Candidates should be aware that misuse of units and/or significant figures, i.e. failure to quote units where necessary, the inclusion of units in quantities defined as ratios or quoting answers to an inappropriate number of significant figures, is liable to be penalised.
SUBJECT CONTENT
Preamble
Students who offer H3 Chemistry should have a strong foundation in H2 Chemistry, through the three core ideas of matter, structure and properties, and transformation, as well as through the extension topics of chemistry of aqueous solutions, organic chemistry, electrochemistry and chemistry of the transition elements.
The syllabus for H3 Chemistry builds on that for H2 Chemistry, and includes the whole of the H2 Chemistry syllabus. Only content that is not already part of the H2 Chemistry syllabus is specifically set out here.
The H3 Chemistry syllabus introduces additional content in two areas, namely Spectroscopic Techniques and Further Organic Mechanisms. The additional content has been selected to highlight basic principles in chemistry and to strengthen the focus on applications. The topics chosen as extensions to the H2 syllabus expand the scope for students to engage in solving challenging problems, while allowing a deeper appreciation of the unity, cohesion and beauty of the discipline of chemistry.
With this expanded scope, students who offer H3 Chemistry are expected to tackle more sophisticated problems than students who only offer H2 Chemistry.
1. SPECTROSCOPIC TECHNIQUES
1.1 Basic Principles of Spectroscopy
Content
• Molecular orbital theory
• Electromagnetic spectrum
• Quantisation of energy
• Energy level transitions
Learning Outcomes
Candidates should be able to:
(a) understand basic molecular orbital (MO) theory, involving
(i) atomic and molecular orbitals
(ii) bonding, anti-bonding and non-bonding orbitals
(iii) molecular orbitals with σ and π symmetry
(b) understand that molecular orbitals represent discrete electronic energy levels in molecules
[see also e(ii)]
(c) apply linear combination of atomic orbitals (LCAO) principles to obtain the shape and relative energies of molecular orbitals in the following:
(i) simple homonuclear diatomic molecules such as H2 , O2 , and F2
(ii) benzene and linear polyenes (molecular orbitals of π symmetry only)
[quantitative treatment of LCAO is not required]
(d) construct and interpret molecular orbital diagrams, and identify the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) for the following:
(i) simple homonuclear diatomic molecules such as H2 , O2 , and F2
(ii) benzene and linear polyenes (molecular orbitals of π symmetry only)
[knowledge of orbital mixing between orbitals of the same symmetry is not required]
(e) understand the following in relation to the fundamental principles of spectroscopy:
(i) properties of electromagnetic radiation
‒ the electromagnetic spectrum (with range of wavelengths for different types of radiation used in spectroscopy)
‒ the photon as a discrete packet (quantum) of electromagnetic energy
‒ the relationship between wavelength, frequency and speed of light, including the use of the
equation, E = hf
(ii) the quantisation of energy in relation to
‒ electronic, vibrational and rotational energy levels
‒ nuclear energy levels in applied magnetic field
(iii) energy level transitions associated with the absorption and emission of photons with energy matching the energy gap
1.2 Ultraviolet/visible Spectroscopy
Content
• Electronic transitions
• Chromophores
• Molar absorptivity and the Beer–Lambert law
Learning Outcomes
Candidates should be able to:
(a) explain that ultraviolet/visible absorption in organic molecules requires electronic transitions ( σ→σ*, n→σ*, π→π*, n→π* transitions; forbidden and allowed transitions) between energy levels in chromophores which contain a double or triple bond, a delocalised system, or a lone pair of electrons
[detailed knowledge of instrumentation is not required]
(b) predict whether a given organic molecule will absorb in the ultraviolet/visible region by identifying the chromophore
(c) explain qualitatively how increasing conjugation in an organic molecule decreases the gap between energy levels and hence shifts the absorption towards longer wavelength
(d) use the Beer–Lambert law, absorbance = lg(Io/I) = εcl , where ε is taken merely as a constant characteristic of the substance concerned, to calculate the concentration of a given species (either organic or inorganic) in solution
(e) apply ultraviolet/visible spectroscopy to quantitative analysis of a given species (either organic or inorganic) in solution
1.3 Infra-red (IR) Spectroscopy
Content
• molecular vibrations: stretching and bending
• characteristic IR absorptions
Learning Outcomes
Candidates should be able to:
(a) explain the origin of IR spectroscopy in simple molecules in terms of
(i) stretching vibrations
(ii) bending vibrations
[detailed knowledge of instrumentation is not required]
(b) predict the number of IR absorptions for a given simple molecule (e.g. CO2 or SO2 ), and identify the molecular vibrations which give rise to them
(c) identify characteristic IR absorptions in the IR spectrum of a compound which may contain different functional groups
[Absorptions of common functional groups will be provided in the Data Booklet.]
(d) suggest structures for a compound from its IR spectrum
(e) predict the characteristic IR absorptions that will be present in the IR spectrum of a compound, given its structure
1.4 Nuclear Magnetic Resonance (NMR) Spectroscopy
Content
• nuclear spin and energy absorption
• chemical shift: δ scale, internal reference, electronegativity effects, anisotropic effects, hydrogen bonding
• calculation of peak area and proton counting
• spin-spin splitting
Learning Outcomes
(a) outline the basic principles of NMR with reference to
(i) nuclear spin
(ii) the process of absorption of energy
[quantitative calculations of transitional energy are not required; detailed knowledge of instrumentation is not required]
(b) understand the following features and use them in the interpretation and prediction of 1H NMR spectra:
(i) chemical shift
(ii) deuterated solvents in the identification of labile protons
(iii) the number of 1H NMR signals: equivalent and non-equivalent protons
(iv) peak area (integration) and proton counting
(v) spin-spin splitting: first order spin-spin coupling; multiplicity
(c) explain the use of the δ scale with tetramethylsilane (TMS) as the reference
(d) explain the factors affecting chemical shift
(i) electronegativity: inductive effect of substituents, including shielding and deshielding effects
(ii) anisotropic effects
(iii) hydrogen bonding
1.5 Mass Spectrometry
Content
• ionisation, fragmentation and mass/charge ratio
• interpretation of spectra: molecular ion peak, isotopic abundance, fragment ions
Learning Outcomes
Candidates should be able to:
(a) outline the basic principles of mass spectrometry, with reference to
(i) ionisation and fragmentation
(ii) mass/charge ratio, m/z
[detailed knowledge of instrumentation is not required]
(b) understand the following features and use them in the interpretation and prediction of mass spectra:
(i) molecular ion peak
(ii) isotopic abundances including the use of (M+1) peak caused by 13C and (M+2) and (M+4) peaks for the identification of halogen compounds
(iii) major fragment ions
[fragment ions obtained from rearrangements are not included]
2. FURTHER ORGANIC MECHANISMS
2.1 Molecular Stereochemistry
Content
• stereochemical projection
• isomerism: conformational, cis-trans , enantiomerism, diastereomerism
Learning Outcomes
Candidates should be able to:
(a) (i) use stereochemical projections, including Newman projections, to represent molecules
(ii) interpret stereochemical projections of molecules
[knowledge of Fischer projections is not required]
(b) apply their understanding of the following types of isomerism to explain the stereochemistry of molecules, including saturated ring systems:
(i) conformational isomerism, including energy barriers to rotation and interconversion
(ii) cis-trans isomerism, including E , Z nomenclature
(iii) enantiomerism and diastereomerism:
‒ R , S configuration
‒ optical activity
‒ optical purity as the excess of one enantiomer, including calculation of optical purity by the equation:
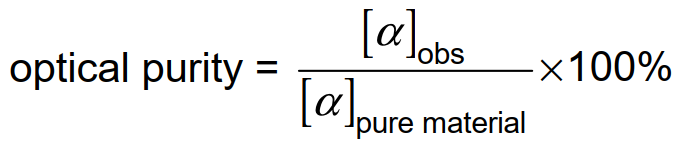
2.2 Basic Physical Organic Chemistry
Content
• kinetic and thermodynamic control: the Hammond postulate, the Bell–Evans–Polanyi principle
• calculations involving activation energy and enthalpy change of reaction
Learning Outcomes
Candidates should be able to:
(a) understand and apply the following concepts involving kinetic control and thermodynamic control to the study of reaction mechanisms:
(i) the Hammond postulate: relationship between the transition state and the nearest stable species
(ii) the Bell–Evans–Polanyi principle
‒ relationship between activation energy and enthalpy change of reaction
‒ quantitative calculations based on Ea = A + BΔHr
2.3 Nucleophilic Substitution
Content
• mechanism: nature of nucleophiles and leaving group, SN1, SN2
• kinetics of mechanisms: energy profile, rate law, simple rate equations, orders of reaction, rate constants, stereochemistry, substituent effects
• competition between SN1 and SN2
Learning Outcomes
Candidates should be able to:
(a) explain how the relative rate of nucleophilic substitution is affected by the nature of the
(i) nucleophile
(ii) leaving group
(iii) substituents
(b) describe and compare the mechanisms and kinetics of SN1 and SN2 reactions, in terms of
(i) the energy profile and rate law, including steady state approximation in SN1
[mathematical treatment of steady state is not required]
(ii) stereochemistry, including ion pair interactions in SN1
(iii) substituent effects
(c) explain the factors affecting competition between SN1 and SN2 mechanisms
[solvent effects are not required]
2.4 Elimination
Content
• mechanism: syn-/anti-elimination, stereoselectivity, regioselectivity, E1, E2
• kinetics of mechanisms: energy profile, rate law, regioselectivity
• E2/SN2 competition: substrate effects, base effects
Learning Outcomes
Candidates should be able to:
(a) understand and apply the following concepts to the study of elimination reactions:
(i) syn - and anti - elimination; and its effect on stereoselectivity
(ii) regioselectivity: Zaitsev (thermodynamic) and Hofmann (kinetic) product(s)
(b) describe and compare the mechanisms and kinetics of E1 and E2 reactions, in terms of
(i) the energy profile and rate law
(ii) regioselectivity
(c) explain the E2/SN2 competition, in terms of
(i) substrate effects
(ii) base effects
SUMMARY OF KEY QUANTITIES AND UNITS
The list below is intended as a guide to the more important quantities which might be encountered in teaching and used in question papers. The list is not exhaustive.
Quantity | Usual symbols | Unit |
Base quantities | ||
amount of substance | n | mol |
electric current | I | A |
length | l | m |
mass | m | kg, g |
thermodynamic temperature | T | K |
time | t | s |
Other quantities | ||
acid dissociation constant | Ka | mol dm–3 |
atomic mass | ma | g, kg |
Avogadro constant | L | mol–1 |
base dissociation constant | Kb | mol dm–3 |
bond energy | – | kJ mol–1 |
charge on the electron | e | C |
concentration | c | mol dm–3 |
density | ρ | kg m–3, g dm–3, g cm–3 |
electric potential difference | V | V |
electromotive force | E | V |
electron affinity | – | kJ mol–1 |
enthalpy change of reaction | ΔH | J, kJ |
equilibrium constant | K, Kp, Kc | as appropriate |
Faraday constant | F | C mol–1 |
frequency | v, f | Hz |
half-life | T½, t½ | s |
ionic product, solubility product | K, Ksp, | as appropriate |
ionic product of water | Kw | mol2 dm–6 |
ionisation energy | I | kJ mol–1 |
lattice energy | – | kJ mol–1 |
molar absorption coefficient | ε | mol–1 dm3 cm–1 |
molar gas constant | R | J K–1 mol–1 |
molar mass | M | g mol–1 |
mole fraction | x | – |
molecular mass | m | g, kg |
neutron number | N | – |
nucleon number | A | – |
number of molecules | N | – |
number of molecules per unit volume | n | m–3 |
order of reaction | n, m | – |
partition coefficient | K | – |
Planck constant | h | J s |
pH | pH | – |
pressure | p | Pa |
proton number | Z | – |
rate constant | k | as appropriate |
relative atomic or isotopic mass | Ar | – |
relative molecular mass | Mr | – |
specific rotation | [ α ] | – |
speed of electromagnetic waves | c | m s–1 |
(standard) electrode or redox potential | (E)θ E | V |
standard enthalpy change of reaction | ΔHθ | J mol–1, kJ mol–1 |
standard entropy change of reaction | ΔSθ | J K–1 mol–1, kJ K–1 mol–1 |
standard Gibbs free energy change of reaction | ΔGθ | J mol–1, kJ mol–1 |
temperature | θ, t | °C |
volume | V, v | m3, dm3 |
wavelength | λ | m, mm, nm |
MATHEMATICAL REQUIREMENTS
It is assumed that candidates will be competent in the techniques described below.
Make calculations involving addition, subtraction, multiplication and division of quantities.
Make approximate evaluations of numerical expressions.
Express small fractions as percentages, and vice versa.
Calculate an arithmetic mean.
Transform decimal notation to power of ten notation (standard form).
Use calculators to evaluate logarithms, squares, square roots, and reciprocals.
Change the subject of an equation. (Most such equations involve only the simpler operations but may include positive and negative indices and square roots.)
Substitute physical quantities into an equation using consistent units so as to calculate one quantity. Check the dimensional consistency of such calculations, e.g. the units of a rate constant k.
Solve simple algebraic equations.
Comprehend and use the symbols/notations <, >, ≈, /, Δ, ≡, x̄ (or <x>).
Test tabulated pairs of values for direct proportionality by a graphical method or by constancy of ratio.
Select appropriate variables and scales for plotting a graph, especially to obtain a linear graph of the form y = mx + c.
Determine and interpret the slope and intercept of a linear graph.
Choose by inspection a straight line that will serve as the ‘least bad’ linear model for a set of data presented graphically.
Understand (i) the slope of a tangent to a curve as a measure of rate of change, (ii) the ‘area’ below a curve where the area has physical significance, e.g. Boltzmann distribution curves.
Comprehend how to handle numerical work so that significant figures are neither lost unnecessarily nor used beyond what is justified.
Estimate orders of magnitude.
Formulate simple algebraic equations as mathematical models, e.g. construct a rate equation, and identify failures of such models.
Calculators
Any calculator used must be on the Singapore Examinations and Assessment Board list of approved calculators.
GLOSSARY OF TERMS
It is hoped that the glossary (which is relevant only to science subjects) will prove helpful to candidates as a guide, i.e. it is neither exhaustive nor definitive. The glossary has been deliberately kept brief not only with respect to the number of terms included but also to the descriptions of their meanings. Candidates should appreciate that the meaning of a term must depend in part on its context.
1. Define (the term(s)...) is intended literally, only a formal statement or equivalent paraphrase being required.
2. What do you understand by/What is meant by (the term(s)...) normally implies that a definition should be given, together with some relevant comment on the significance or context of the term(s) concerned, especially where two or more terms are included in the question. The amount of supplementary comment intended should be interpreted in the light of the indicated mark value.
3. State implies a concise answer with little or no supporting argument, e.g. a numerical answer that can be obtained ‘by inspection’.
4. List requires a number of points, generally each of one word, with no elaboration. Where a given number of points is specified, this should not be exceeded.
5. Explain may imply reasoning or some reference to theory, depending on the context.
6. Describe requires candidates to state in words (using diagrams where appropriate) the main points of the topic. It is often used with reference either to particular phenomena or to particular experiments. In the former instance, the term usually implies that the answer should include reference to (visual) observations associated with the phenomena.
In other contexts, describe and give an account of should be interpreted more generally, i.e. the candidate has greater discretion about the nature and the organisation of the material to be included in the answer. Describe and explain may be coupled in a similar way to state and explain.
7. Discuss requires candidates to give a critical account of the points involved in the topic.
8. Outline implies brevity, i.e. restricting the answer to giving essentials.
9. Predict implies that the candidate is not expected to produce the required answer by recall but by making a logical connection between other pieces of information. Such information may be wholly given in the question or may depend on answers extracted in an early part of the question.
10. Deduce is used in a similar way as predict except that some supporting statement is required, e.g. reference to a law/principle, or the necessary reasoning is to be included in the answer.
11. Comment is intended as an open-ended instruction, inviting candidates to recall or infer points of interest relevant to the context of the question, taking account of the number of marks available.
12. Suggest is used in two main contexts, i.e. either to imply that there is no unique answer (e.g. in chemistry, two or more substances may satisfy the given conditions describing an ‘unknown’), or to imply that candidates are expected to apply their general knowledge to a ‘novel’ situation, one that may be formally ‘not in the syllabus’.
13. Find is a general term that may variously be interpreted as calculate, measure, determine, etc.
14. Calculate is used when a numerical answer is required. In general, working should be shown, especially where two or more steps are involved.
15. Measure implies that the quantity concerned can be directly obtained from a suitable measuring instrument, e.g. length, using a rule, or angle, using a protractor.
16. Determine often implies that the quantity concerned cannot be measured directly but is obtained by calculation, substituting measured or known values of other quantities into a standard formula, e.g. relative molecular mass.
17. Estimate implies a reasoned order of magnitude statement or calculation of the quantity concerned, making such simplifying assumptions as may be necessary about points of principle and about the values of quantities not otherwise included in the question.
18. Sketch, when applied to graph work, implies that the shape and/or position of the curve need only be qualitatively correct, but candidates should be aware that, depending on the context, some quantitative aspects may be looked for, e.g. passing through the origin, having an intercept, asymptote or discontinuity at a particular value.
In diagrams, sketch implies that a simple, freehand drawing is acceptable: nevertheless, care should be taken over proportions and the clear exposition of important details.
19. Construct is often used in relation to chemical equations where a candidate is expected to write a balanced equation, not by factual recall but by analogy or by using information in the question.
20. Compare requires candidates to provide both the similarities and differences between things or concepts.
21. Classify requires candidates to group things based on common characteristics.\
22. Recognise is often used to identify facts, characteristics or concepts that are critical (relevant/appropriate) to the understanding of a situation, event, process or phenomenon.
TEXTBOOKS AND REFERENCES
Teachers and students may find reference to the following books helpful.
A Primer to Mechanism in Organic Chemistry by P Sykes, published by Longman Scientific & Technical
Advanced Organic Chemistry (5th Edition) by F A Carey and R J Sundberg, published by Springer
Introduction to Spectroscopy (5th Edition) by D L Pavia, G M Lampman, G S Kriz and J A Vyvyan, published by Cengage Learning
IR Spectroscopy: An Introduction by H Günzler and H Gremlich, published by Wiley-VCH
March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (7th Edition) by M Smith, published by Wiley
Modern Physical Organic Chemistry by E V Anslyn and D A Dougherty, published by University Science
NMR Spectroscopy: Basic Principles, Concepts and Applications in Chemistry (3rd Edition) by H Günther, published by Wiley-VCH
Organic Mechanisms: Reactions, Stereochemistry and Synthesis (English edition) by R Bruckner,
M Harmata and K Beifuss, published by Springer
Organic Spectroscopy by L D S Yadav, published by Kluwer
Organic Synthesis: The Disconnection Approach (2nd Edition) by S Warren and P Wyatt, published by Wiley
Oxford Chemistry Primers: Foundations of Organic Chemistry by M Hornby and J Peach, published by Oxford University Press
Oxford Chemistry Primers: Structure and Reactivity in Organic Chemistry by H Maskill, published by Oxford University Press
Oxford Chemistry Primers: Mechanisms of Organic Chemistry by H Maskill, published by Oxford University Press
Perspectives on Structure and Mechanism in Organic Chemistry (2nd Edition) by F A Carroll, published by Wiley
The Art of Writing Reasonable Organic Reaction Mechanisms (2nd Edition) by R B Grossman, published by Springer
UV Spectroscopy: Techniques, Instrumentation and Data handling by B J Clark, T Frost and M A Russell, published by Chapman & Hall
The Language of Mathematics in Science: A Guide for Teachers of 11–16 (2016) Science by R Boohan, published by the Association for Science Education ISBN 9780863574559 www.ase.org.uk/mathsinscience
Teachers are encouraged to choose texts for class use which they feel will be of interest to their students and will support their own teaching style.
