Structural Elucidation 1
Structural Elucidation questions are usually quite challenging as it requires students to be familiar with all the organic reactions in syllabus and identify the unknown compounds.
We need a very systematic approach to deduce the observations and identify the compounds.
Let's look at this example:
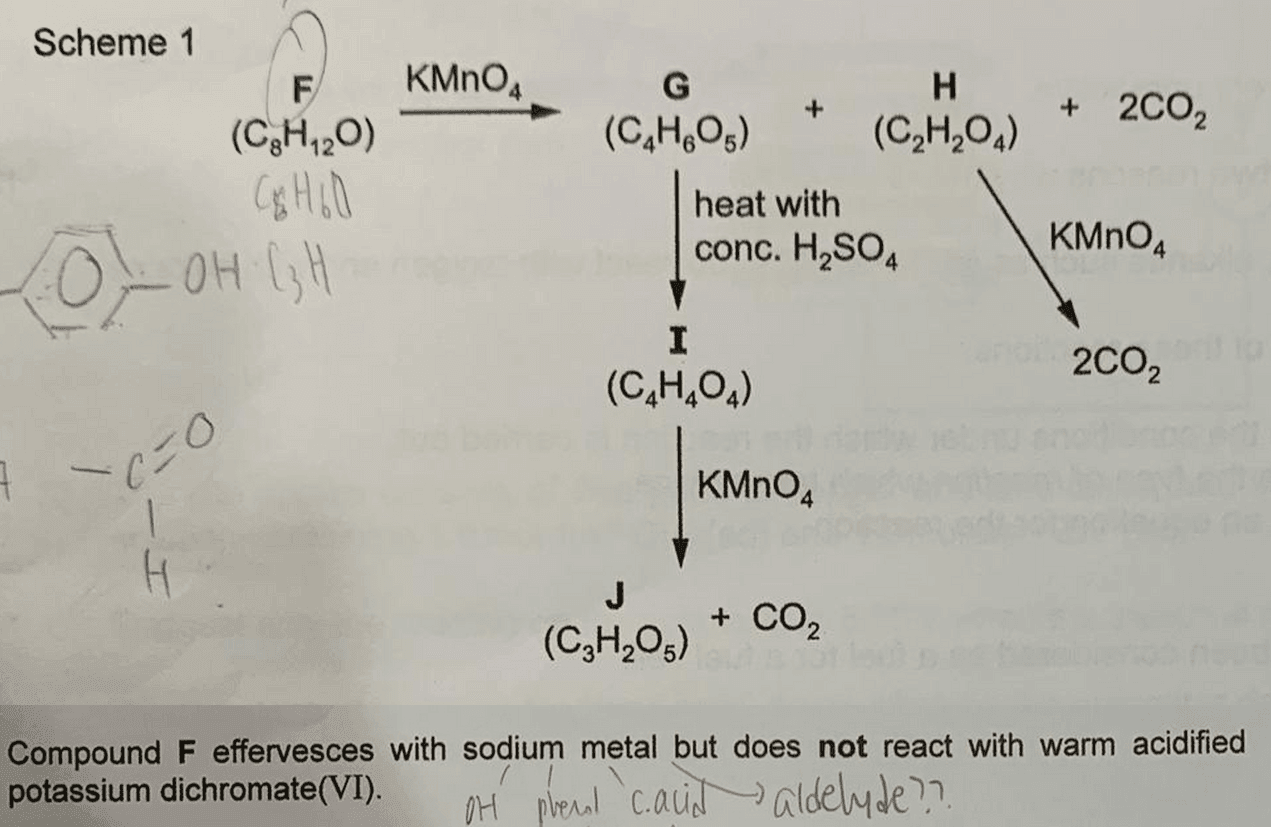
We have a few unknown compounds (from F to J) to identify, so let's do this part by part.
Deduce Functional Groups in F (C8H12O)
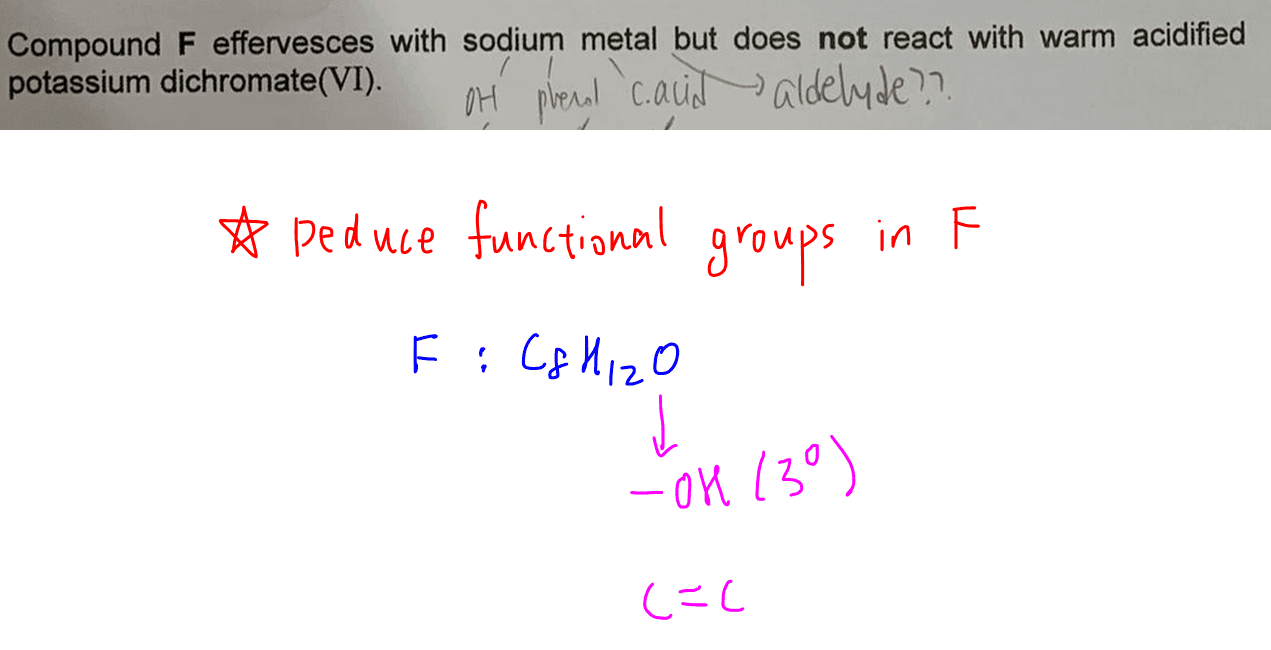
F undergoes acid base reaction with sodium to form H2 gas, so either alcohol or phenol is present (acid is eliminated since F has only 1 oxygen).
F cannot be oxidised by dichromate so it'll have either a tertiary alcohol or phenol functional group as both cannot be oxidised.
F has a close C to H ratio, which suggests a high degree of unsaturation in F.
This is also confirmed in the reaction of F by KMnO4 to form G, H and CO2.
Hence alkene functional groups are present in F.
Also, phenol is rejected since it does not undergo oxidative cleavage and the oxygen in F is due to a tertiary alcohol.
Deduce Functional Groups in G (C4H6O5) and H (C2H2O4)

F undergoes oxidative cleavage with KMnO4 to form G, H and 2 CO2.
Products of oxidation have to be stable to oxidation and products of oxidative cleavage are usually ketone (1 oxygen), acid (2 oxygen) and CO2.
This means we can deduce the number and type of functional groups in G and H.
G has 5 oxygens so should have 2 acid groups and 1 ketone.
However recall there is a tertiary alcohol in F which is resistant to oxidation, hence it will not be oxidised by KMnO4 and that odd oxygen should be due to tertiary alcohol instead of ketone.
Hence G has 2 acid groups and 1 tertiary alcohol group.
H has 4 oxygens so should have 2 acid groups.
Deduce H (C2H2O4)
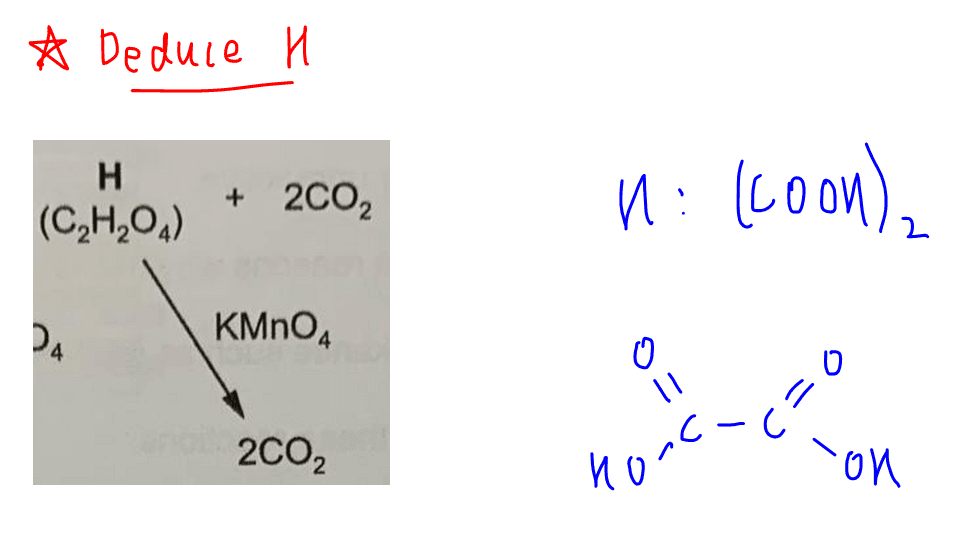
H can be further oxidised to CO2 and this means H must be ethanedioic acid (COOH)2 which is one of the 2 acids in A Level Chemistry syllabus that can be oxidised to CO2 and H2O.
The other acid that can be oxidised to CO2 and H2O is methanoic acid, HCOOH.
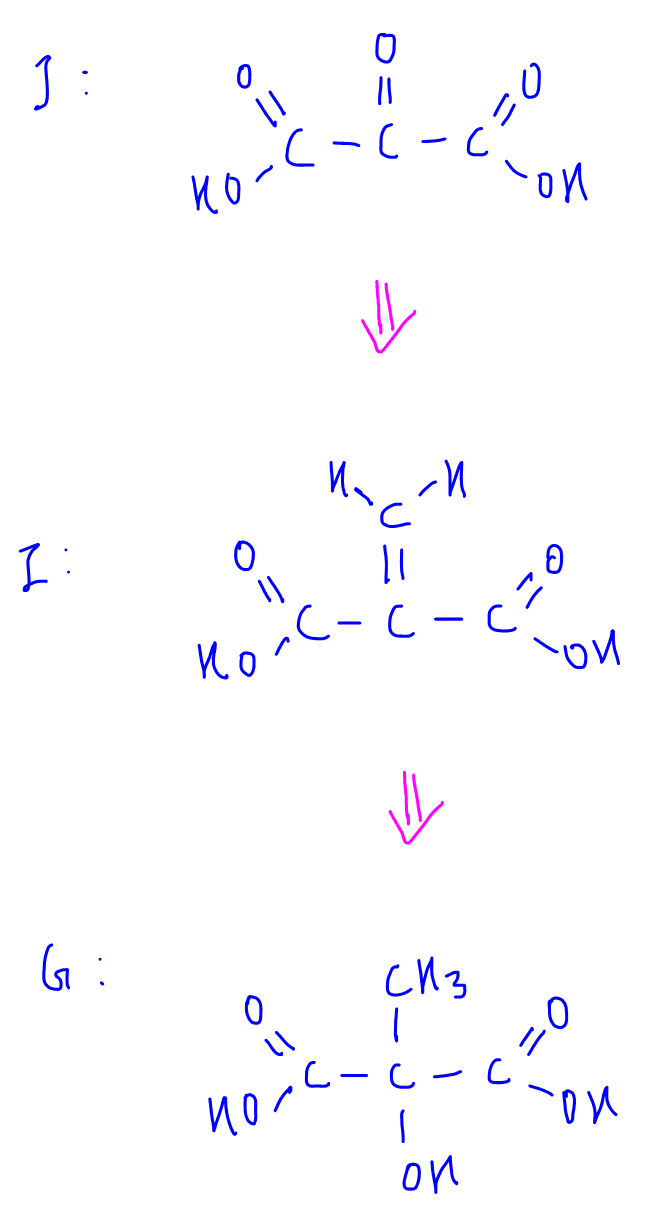
Deduce J (C3H2O5), I (C4H4O4) and G (C4H6O5)
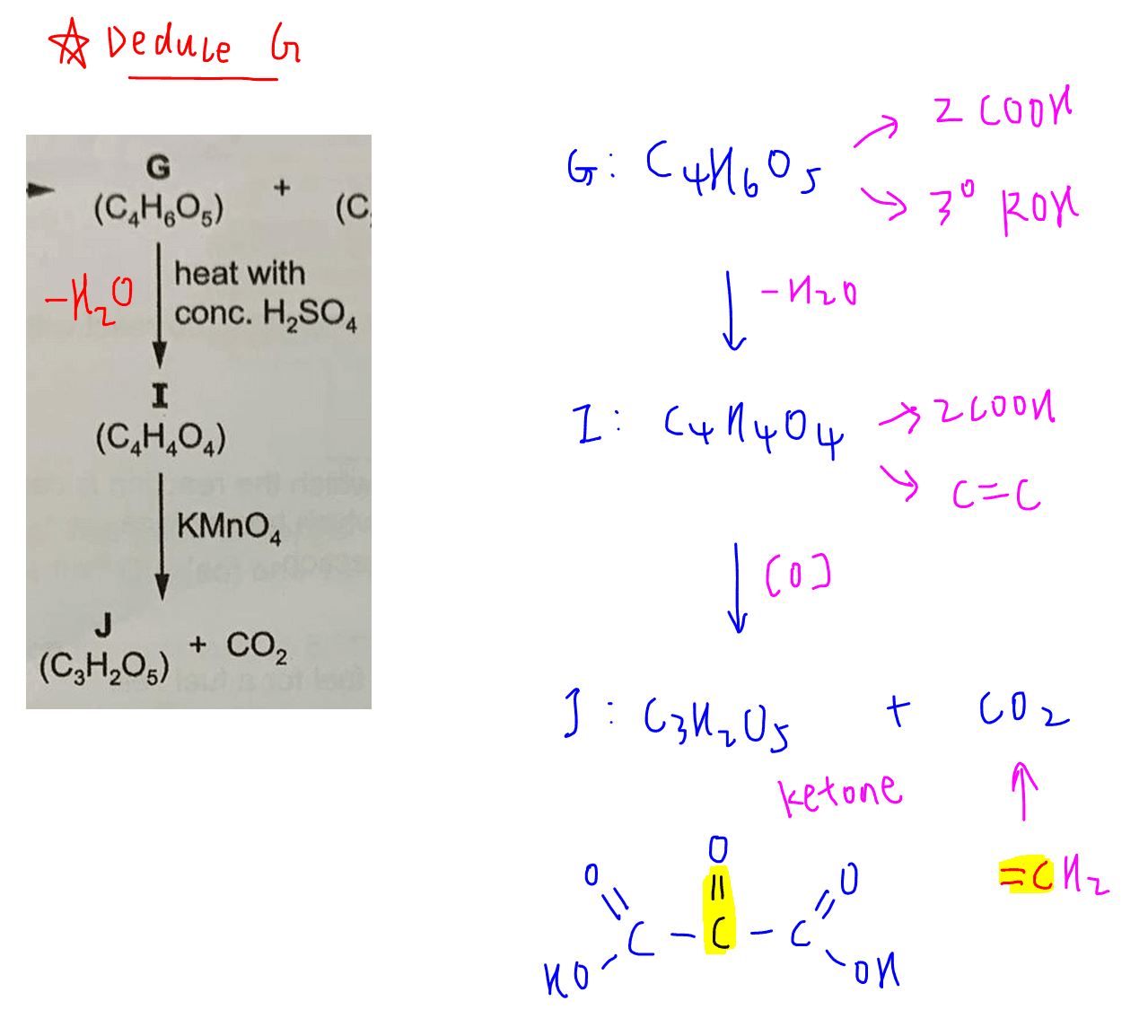
G reacts with conc H2SO4 to form I (C4H4O4).
Comparing molecular formula we can deduce this is an elimination of H2O from tertiary alcohol in G to form alkene in I.
I undergoes oxidative cleavage with KMnO4 to form J (C3H2O5) and CO2.
The additional oxygen in J should be due to a ketone hence we can draw out the structure for J (HOOCCOCOOH).
The CO2 evolved is due to a terminal alkene carbon (CH2=)
So we can work backwards to figure out the structure of I and G.
Remember G has a tertial alcohol so we have to put the -OH group on the tertiary carbon instead of methyl carbon.
Deduce F (C8H12O)
Now we can list down the products of oxidative cleavage of F and deduce the different parts of alkene that will form F.
A simple way to figure this out is to remove the oxygen from the products of oxidative cleavage.
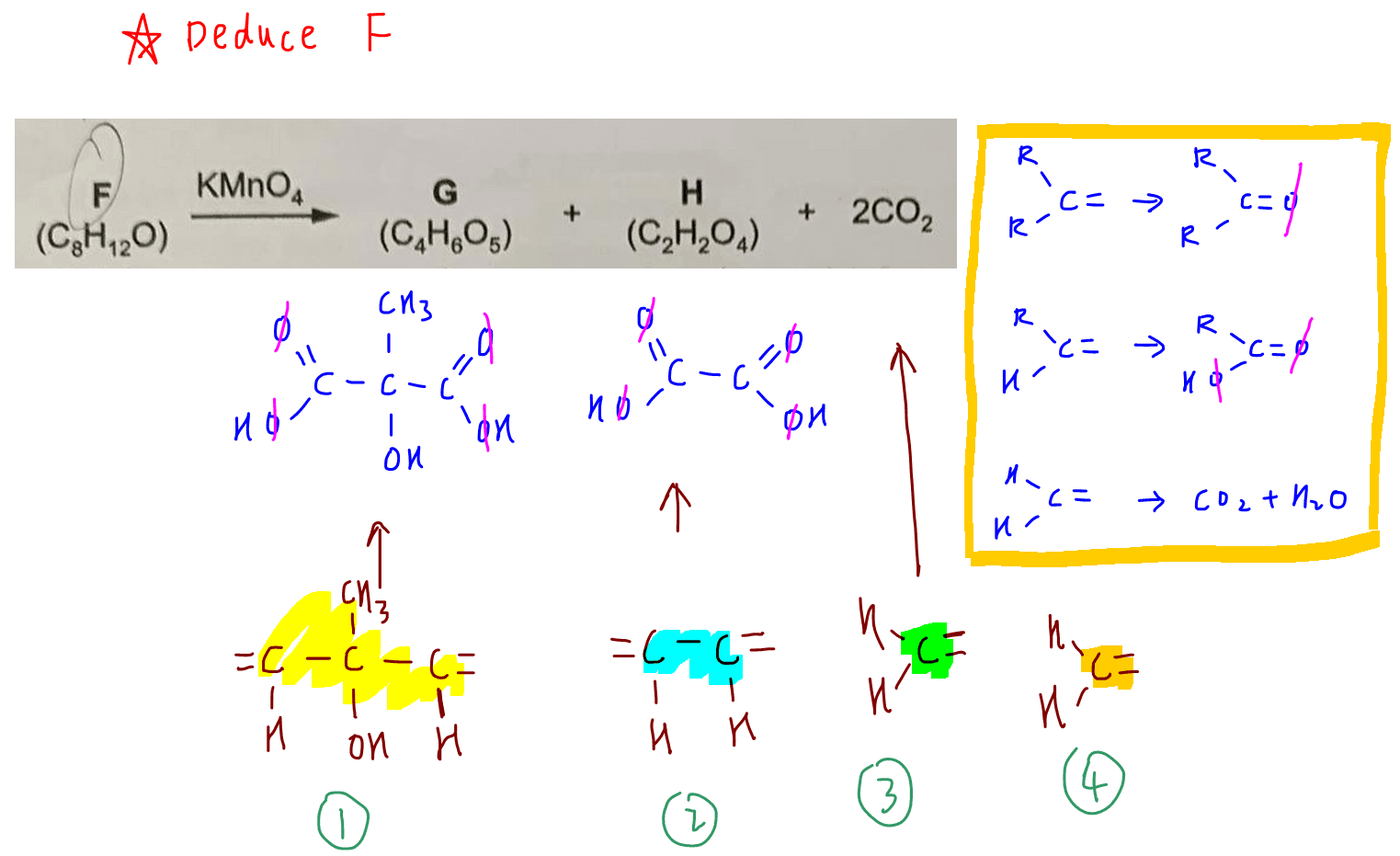
Now we have 4 fragments that we need to piece together.
Thankfully fragments 1 and 2 are symmetrical, which means that putting everything together:
3-1-2-4 and 3-2-1-4 are the same compound F.
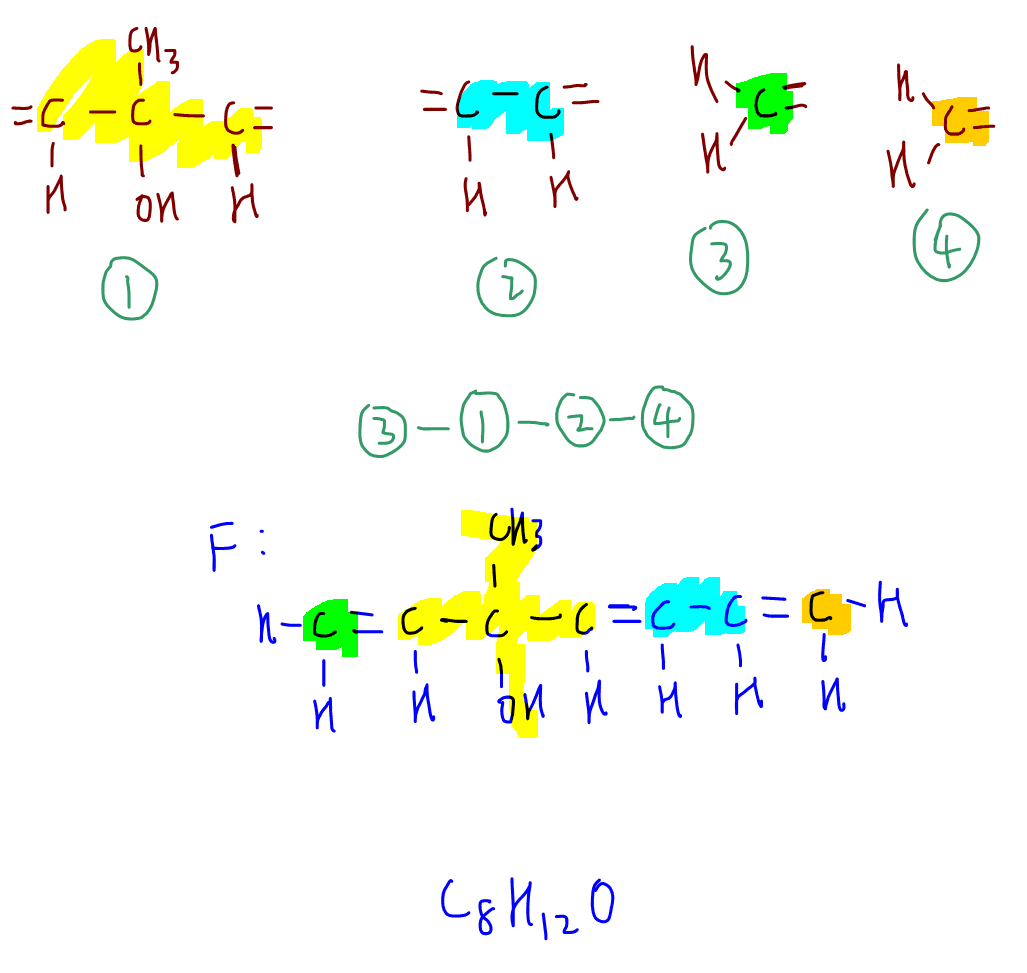
It's always a good idea to verify our answers by checking the molecular formula given in the question.
Topic: Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online tuition classes!
