Theoretical vs Experimental Lattice Energy
In this JC2 webinar we want to compare theoretical and experimental lattice energy.
Let Chemistry Guru, Singapore's renowned JC Chemistry tuition centre, guide you through this question.

We are asked to explain the differences between the experimental and theoretical values of lattice energy for AgF and AgI.
Experimental or actual lattice energy is determined from the Born-Haber cycle of an ionic compound.
Check out this video if you are interested to know how to draw Born-Haber cycle.
Theoretical or calculated lattice energy is based on the following formula assuming a highly ionic compound.
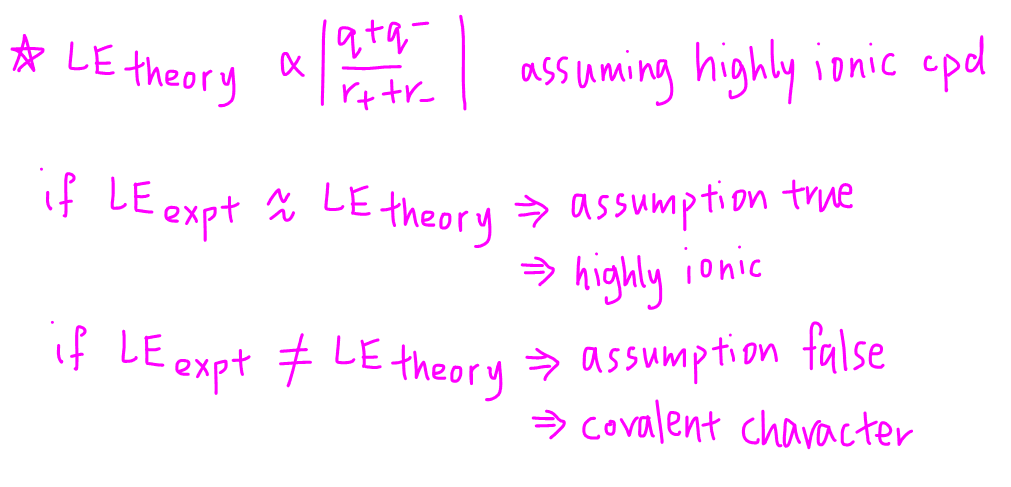
A highly ionic compound has only electrostatic attraction between cations and anions.
There is no distortion of electron clouds of the anions and no covalent character.
Therefore the difference between theoretical and experimental lattice energy tells us the extent of covalent character in that ionic compound.
If LE(experimental) is very close to LE(theoretical), the assumption that the ionic compound is highly ionic is true.
Therefore the ionic compound is highly ionic with little or no covalent character.
Ionic compounds that are highly ionic consist of:
- cations with low charge density and polarising power
- anions that are small and non-polarisable
If LE(experimental) is significantly different from LE(theoretical), the assumption that the ionic compound is highly ionic is false.
Therefore the ionic compound has significant covalent character.
Ionic compounds that have covalent character consist of:
- cations with high charge density and polarising power
- anions that are large and polarisable
Comparing AgF and AgI, larger iodide ion is more polarisable and there is a greater distortion of electron cloud of iodide.
AgI will have more significant covalent character than AgF hence a greater difference between theoretical and experimental lattice energy.

Topic: Energetics, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's trusted JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
