Thermal Decomposition of Group 2 Metal Salts
In this video created by Chemistry Guru, Singapore's leading JC Chemistry tuition centre, we want to explain the trends that we observe for thermal decomposition temperatures for Group 2 Metal Salts.
Thermal Decomposition Temperatures for Carbonates, Nitrates and Hydroxides
Let's have a few examples.
Here's the decomposition reaction for Group 2 metal carbonates and the temperatures that the salts decompose.
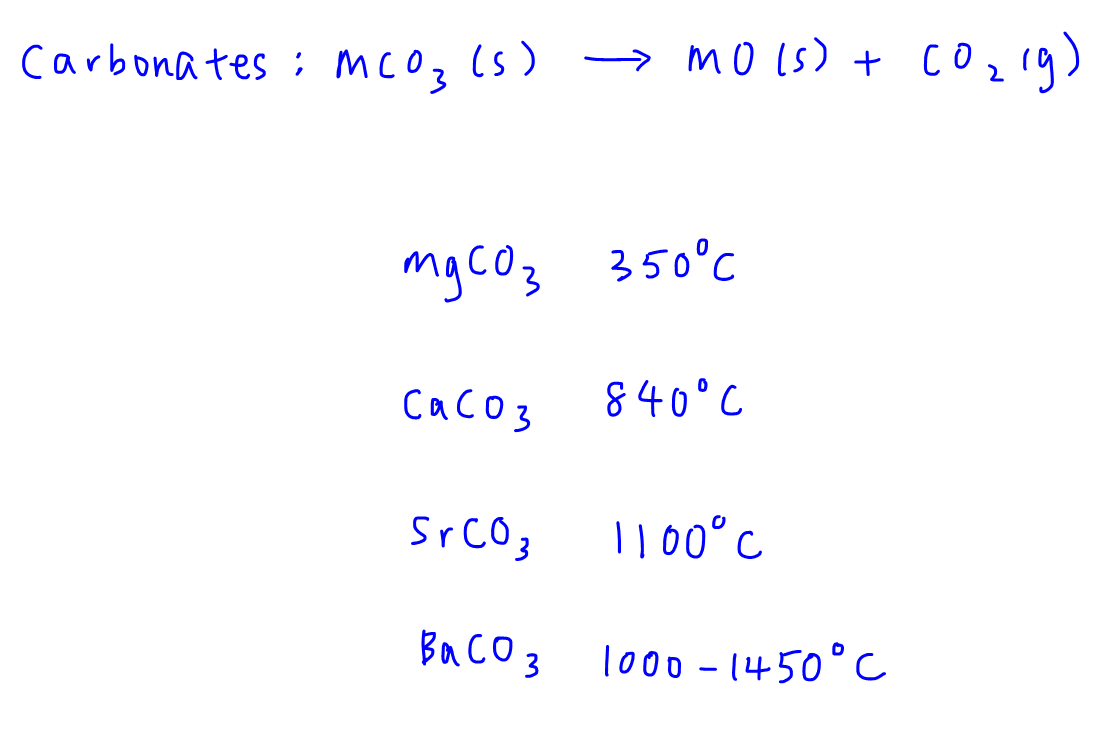
We notice that bonds within the carbonate anion are broken to form metal oxide and carbon dioxide.
We also see that the decomposition temperatures increase from MgCO3 to BaCO3.
For nitrates we notice the same trend.
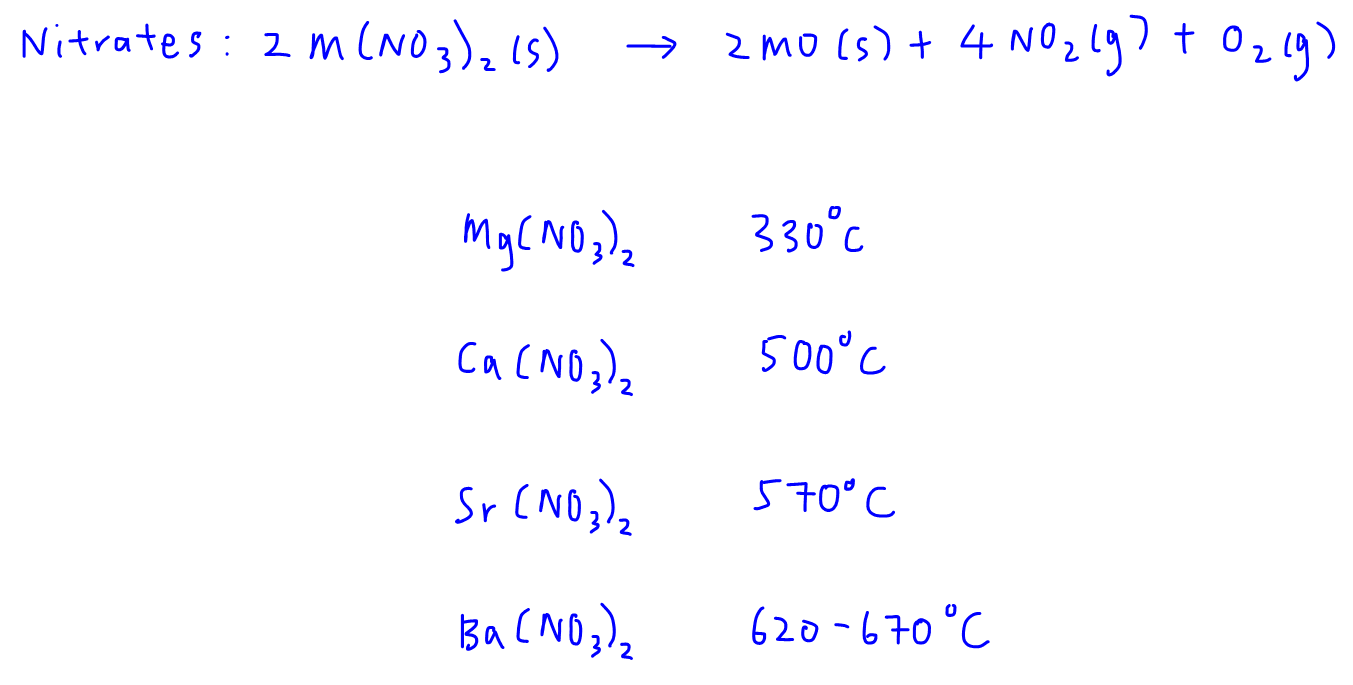
A higher temperature is required to decompose Ba(NO3)2 as compared to Mg(NO3)2.
Even for hydroxides we have the same observations.
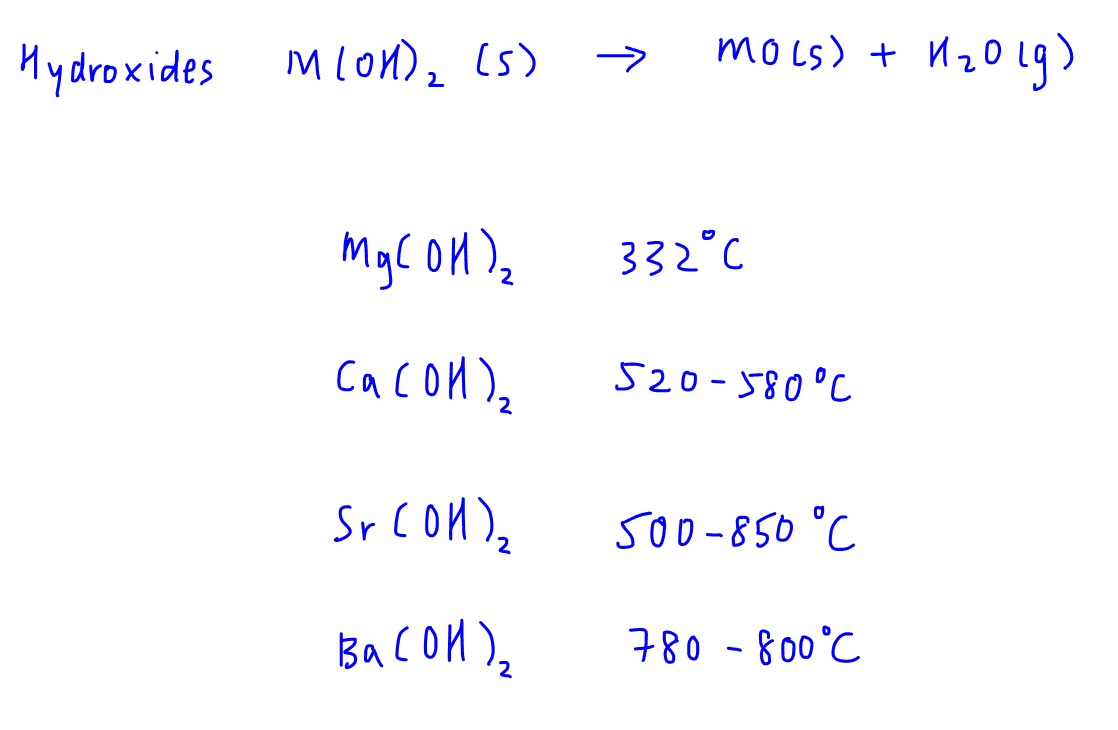
The increasing thermal stability of Group 2 metal salts is consistently seen. So what causes this trend?
Charge Density and Polarising Power of Group 2 Metal Cations
Let's use MgCO3 as an example.
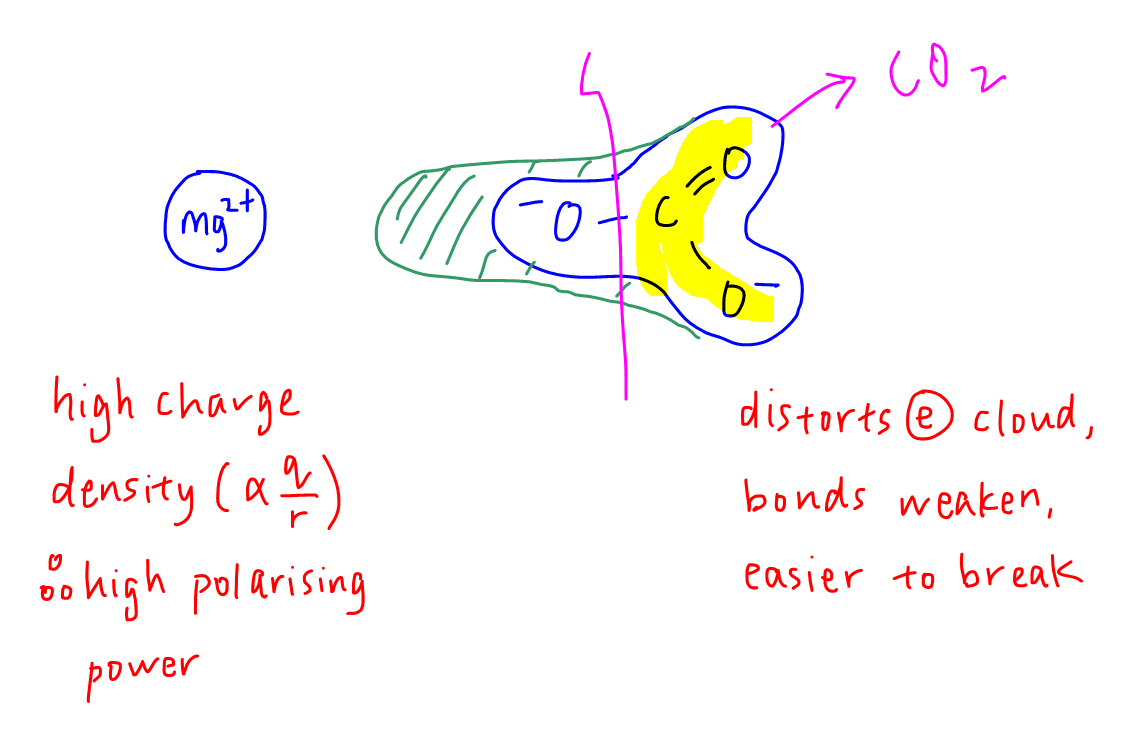
Mg2+ has a small size and high charge so its charge density is considered high in Group 2.
Therefore it has a high polarising power and a strong ability to distort the electron cloud of a neighbouring anion, CO32- in this case.
When the electron cloud of carbonate is distorted, the bonds within the anion will weaken and less energy is required to break the C-O bonds to release CO2.
Therefore the thermal decomposition temperature is lower or the salt is thermally less stable to heat.
Explaining Thermal Decomposition Temperature Trend for Group 2 Metal Salts
Finally we can explain the thermal stability trend for Group 2.
Down the Group the size of metal cation increases, hence charge density and polarising power decreases.
The electron cloud of anion will be distorted to a smaller extent and bonds within the anion are weakened to a smaller extent.
This means the salt will be more stable to heat and require a higher temperature to decompose.

For the detailed step-by-step discussion on how to explain the thermal decomposition trend for Group 2 metal salts, check out this video!
Topic: Group 2 and 17 Elements, Inorganic Chemistry, A Level Chemistry, Singapore
Back to other previous Inorganic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's reputable A Level Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online chemistry classes!
