Titration Curve of Amino Acid
Let Chemistry Guru, Singapore's top JC Chemistry tuition centre, guide you through this question where we need to sketch the titration curve when amino acid alanine at pH=1 is titrated with NaOH.
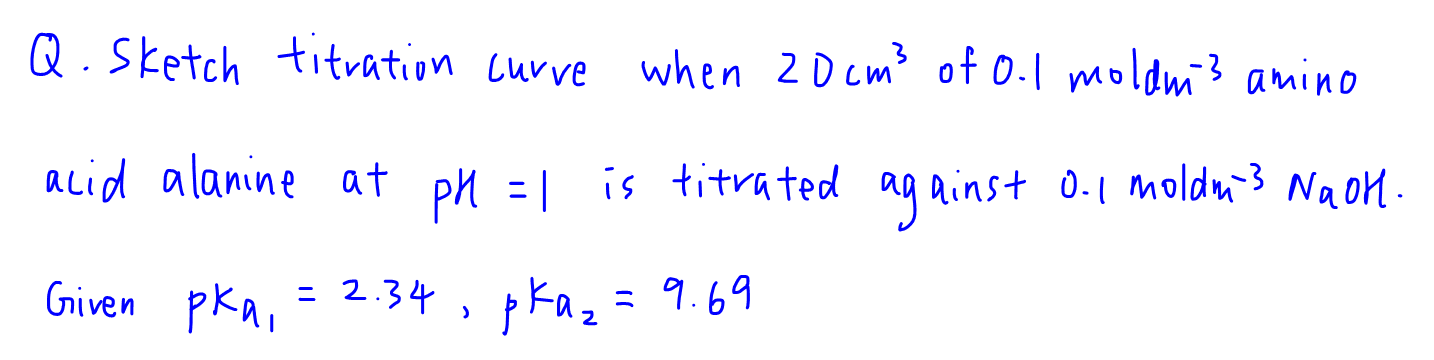
Since the pKa values of alanine is given, we can assign these values to the alpha acid (pKa=2.34) and alpha amino (pKa=9.69) groups first.
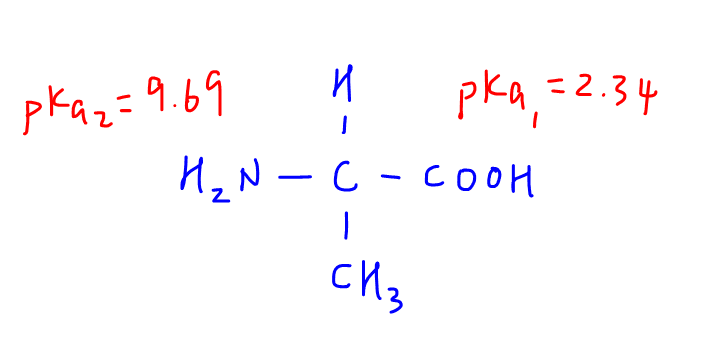
Deduce Structure at pH=1
Next we need to deduce what alanine would exist as at pH=1.
pH 1 will be acidic with respect to the alpha acid group hence it would remain unchanged as an acid.
pH 1 is also acidic with respect to the alpha amino group and it would be converted to conjugate acid.
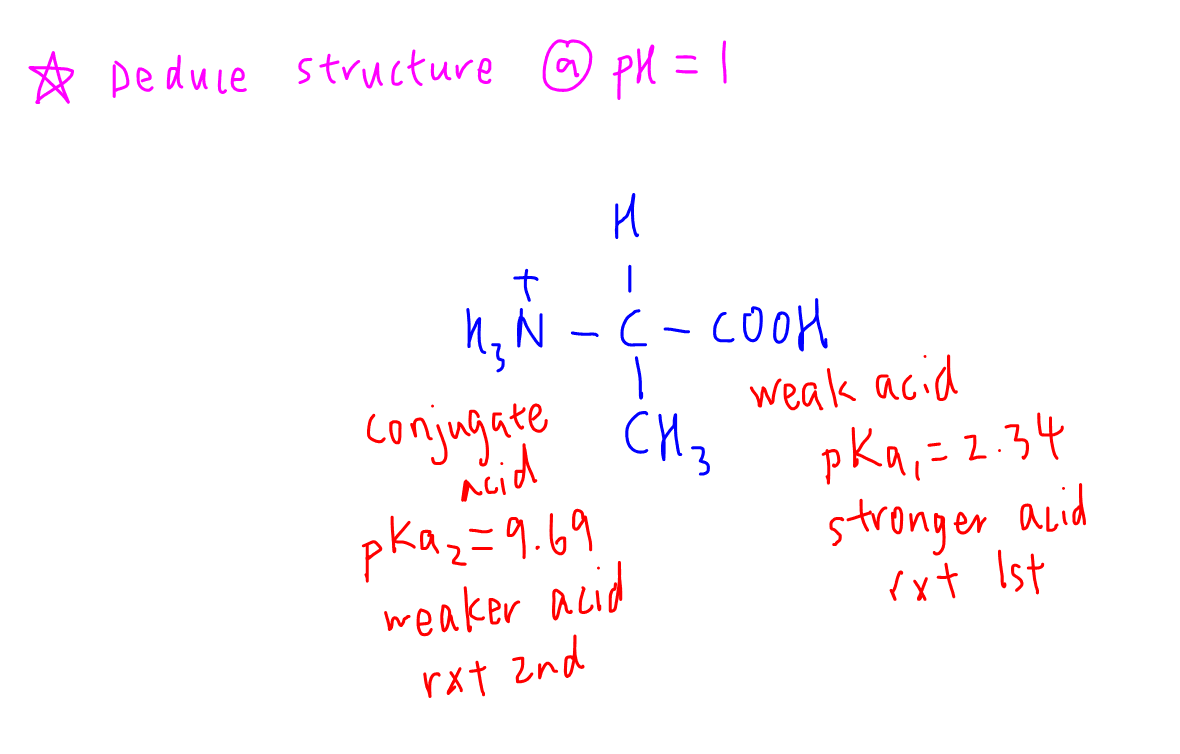
Notice now this structure has now 2 acidic groups, hence it is considered as a diprotic acid.
Diprotic acid will react with NaOH in 2 stages.
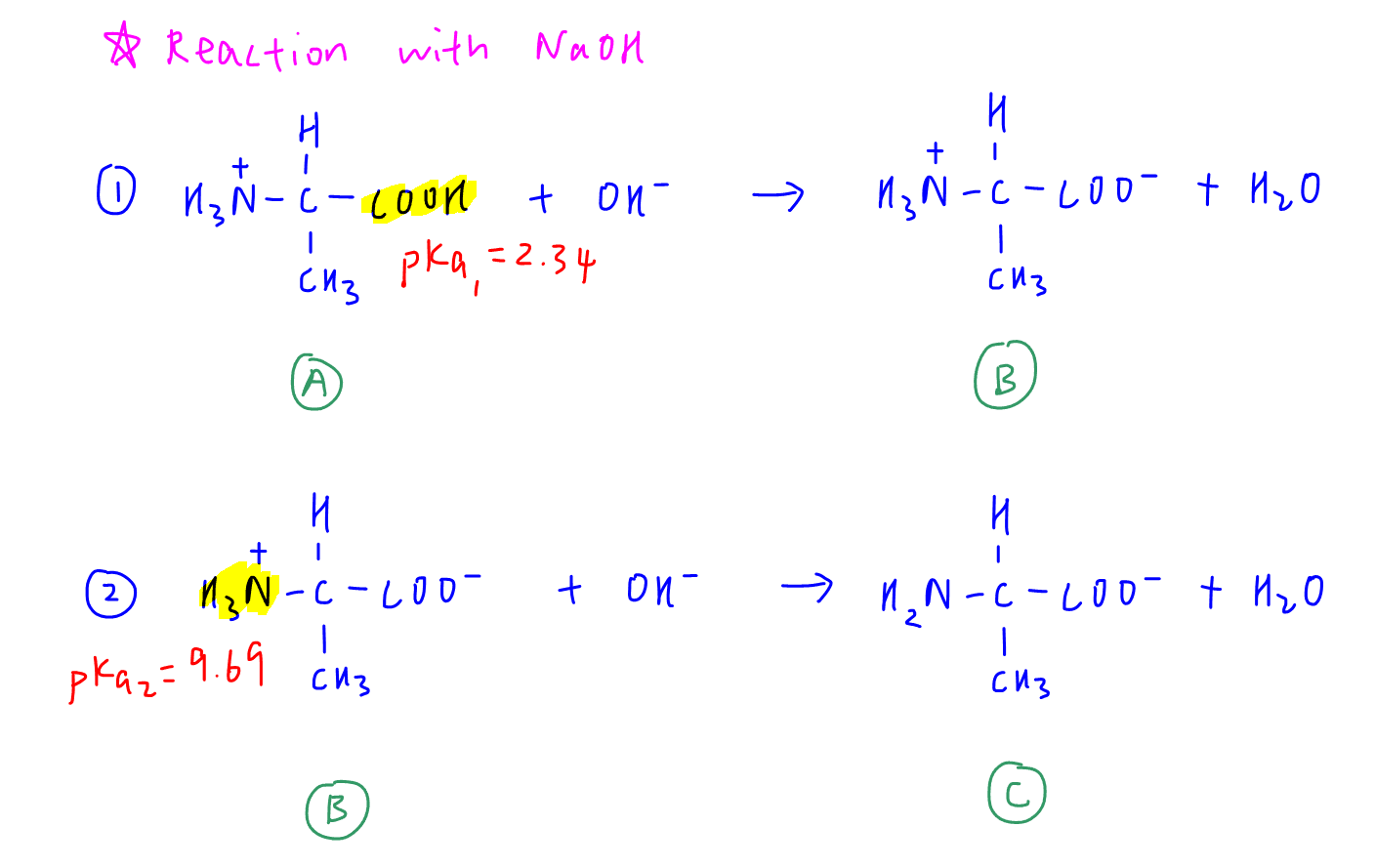
The stronger acid (alpha acid with smaller pKa) will react first and at the end of this distinct reaction, we have a distinct equivalence point.
The weaker acid (alpha ammonium with bigger pKa) will react second and at the end of reaction 2, there is another distinct equivalence point.
Hence we will see 2 distinct equivalence points in the titration curve of alanine with NaOH.
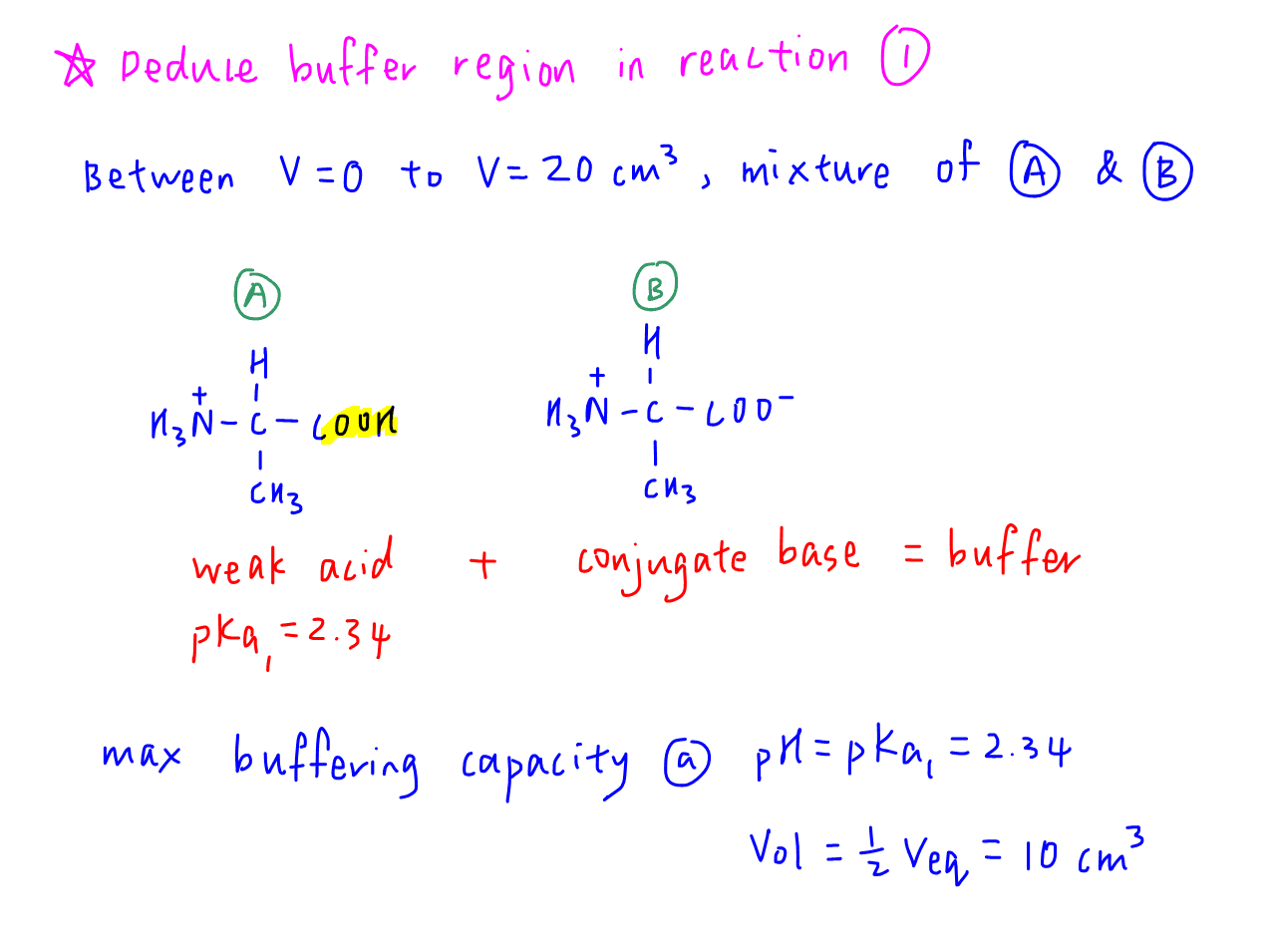
Deduce Buffer Region in Reaction 1
During reaction 1, we have a mixture of structures A and B which are a conjugate acid-base pair.
Therefore this is a buffer solution and we can determine the maximum buffering capacity at:
pH = pKa1 = 2.34
Vol = 0.5 Veq = 10 cm3
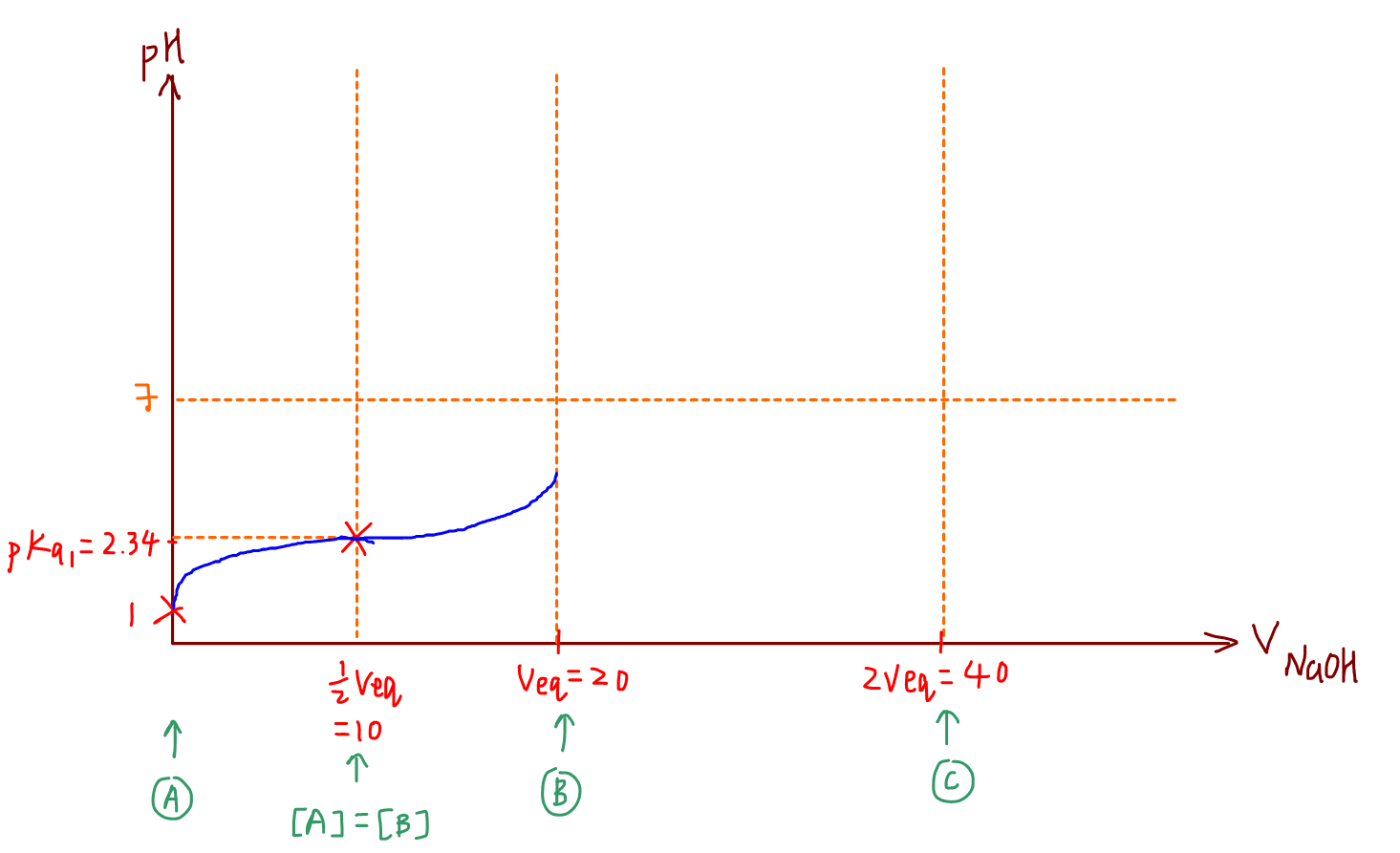
Maximum buffering capacity is a point we can plot on the titration curve and the gradient at that point is zero.
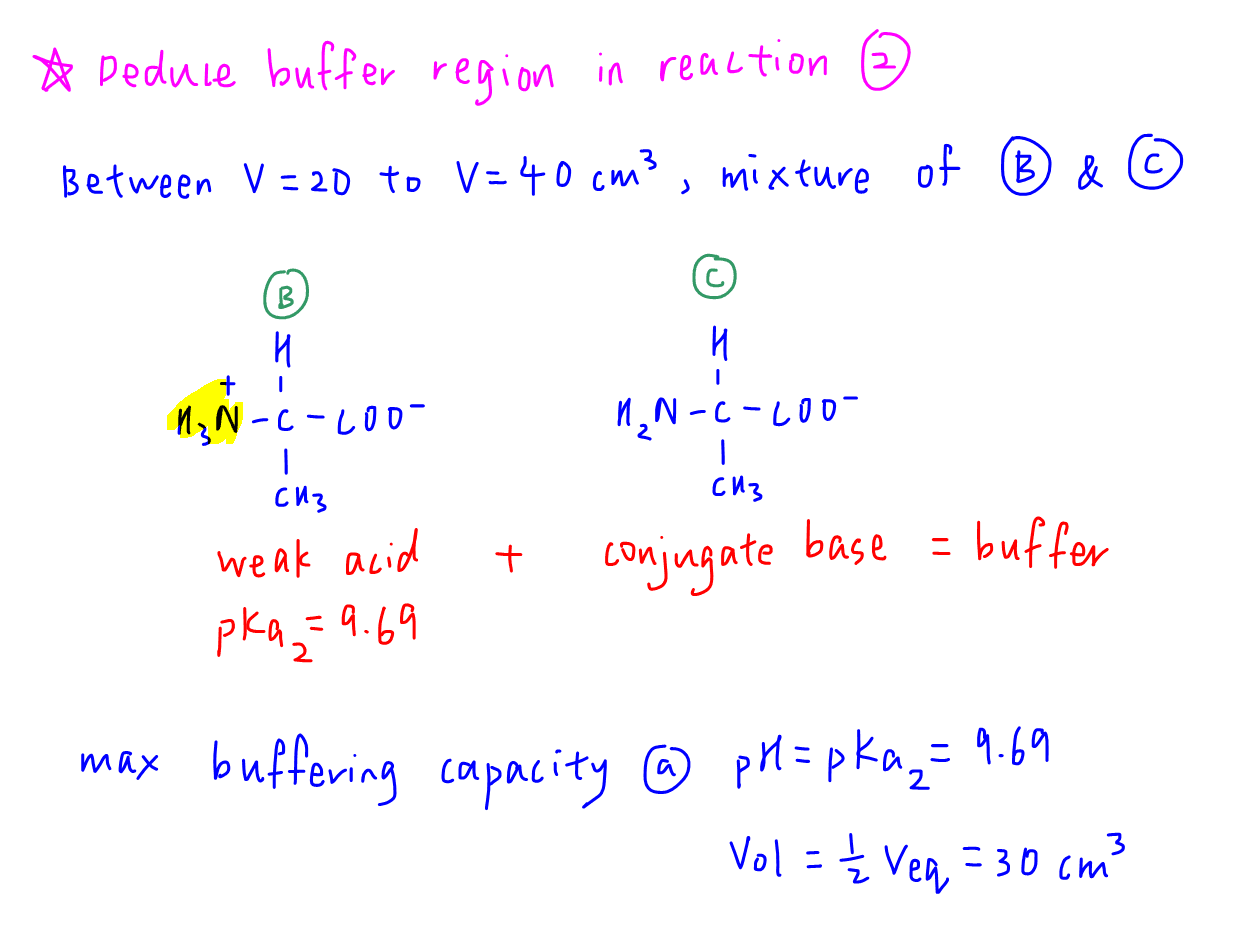
Deduce Buffer Region in Reaction 2
Similarly, there is a buffer solution involving mixture of conjugate acid-base pair B and C in the second reaction.
Maximum buffering capacity for the second buffer is at:
pH = pKa2 = 9.69
Vol = 0.5 Veq = 30 cm3
We can then plot the second mbc and deduce the rest of the titration curve.
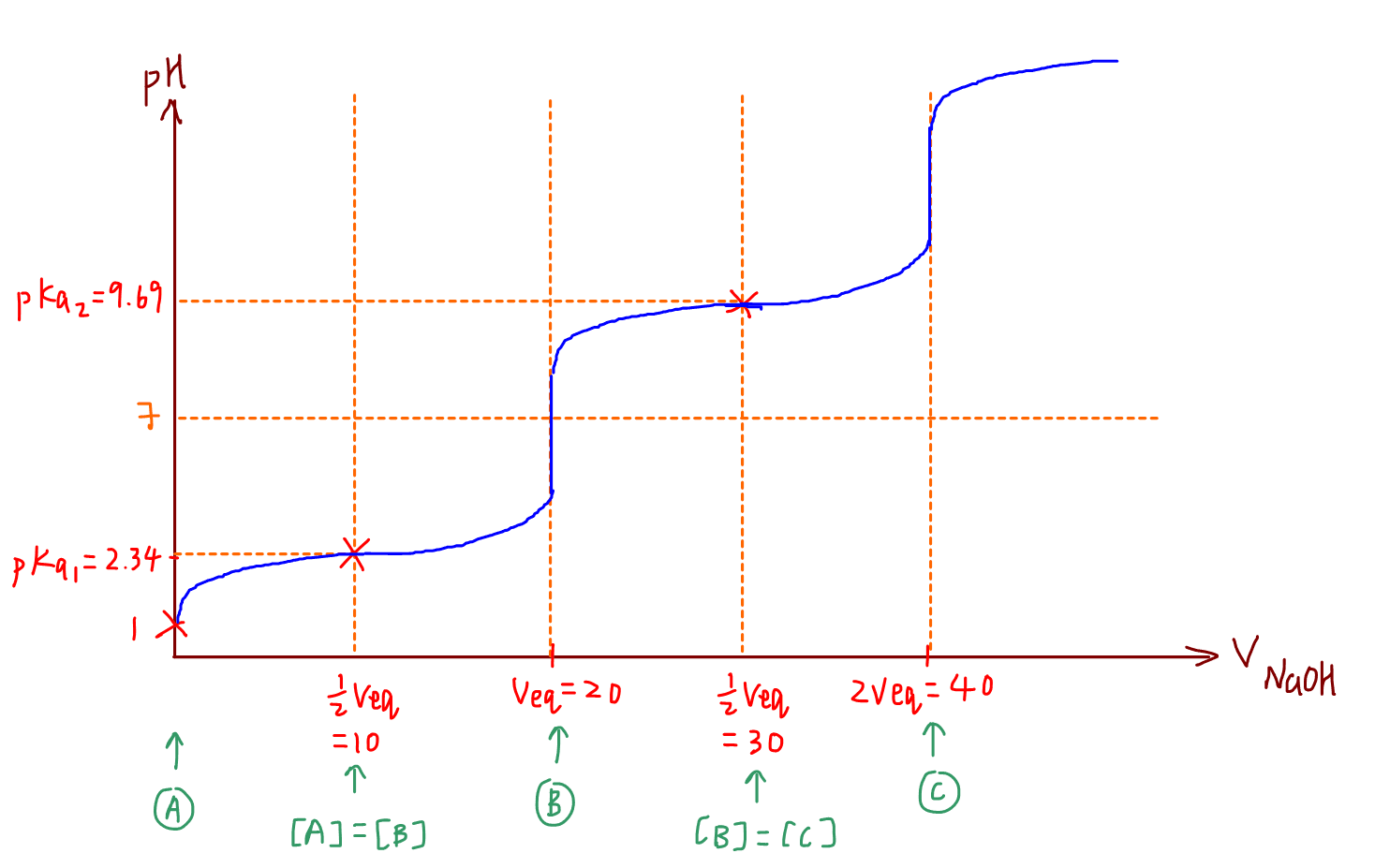
Remember for titration curve involving amino acids and other diprotic acids, the maximum buffering capacities are at pKa1 and pKa2.
These 2 points are very easy to plot and they are well spaced out, so the titration curve will look really nice.
Topic: Nitrogen Compounds, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online tuition classes!
