Transition Metals as Homogeneous Catalysts
Transition metals can function as both homogeneous and heterogeneous catalysts.
Let's focus on homogeneous catalysis in this video created by Chemistry Guru, Singapore's leading JC Chemistry tuition centre.
In homogeneous catalysis, the catalyst is in the same physical state as reactants.
Transition metals can act as homogeneous catalysts due to their variable oxidation states.
This allows the transition metal to act as a medium to transfer electrons between reactants.
Our example is the reaction between S2O82- and I- via the following equation:
S2O82-(aq) + 2I-(aq) → 2SO42-(aq) + I2(aq)
Both reactants are negatively charged and they repel each other.
Activation energy for the reaction is high hence reaction is slow or kinetically less feasible.
This reaction can be catalysed by either Fe2+(aq) or Fe3+(aq).
To deduce the mechanism for catalysed reaction, it's useful to list down the half equations and E values for the reactants and catalyst.
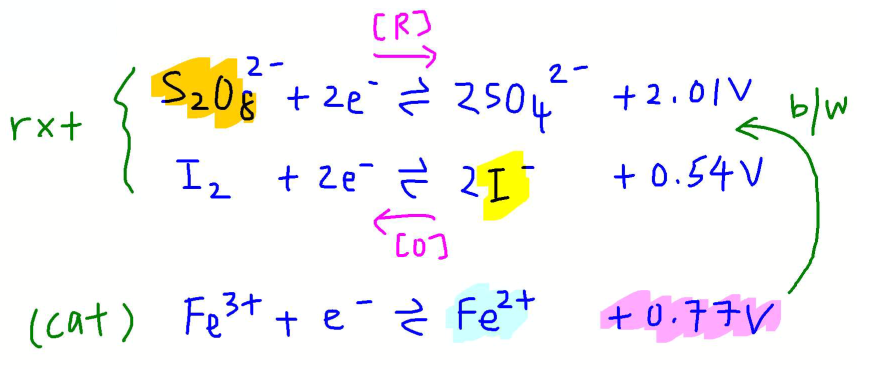
Notice the 2 points that are important to chose a suitable catalyst:
1. Species on both sides of half equation for catalyst are cations (usually M3+ and M2+) so they can attract the negative reactants.
2. E value for catalyst is between E values for reactants. In this case E(Fe3+/Fe2+) = +0.77V is between E(S2O82-/SO42-) = +2.01V and E(I2/I-) = +0.54V.
In step 1, catalyst Fe2+ is oxidised to Fe3+ and we pair this up with reduction of S2O82-.
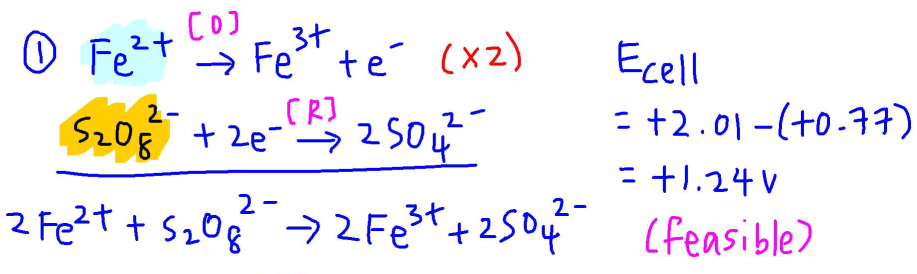
We can balance the equation and show Ecell for step 1 is positive hence feasible.
In step 2, Fe3+ is reduced, Fe2+ is regenerated and we pair this up with oxidation of I-.

Again we can show that Ecell for step 2 is positive and feasible.
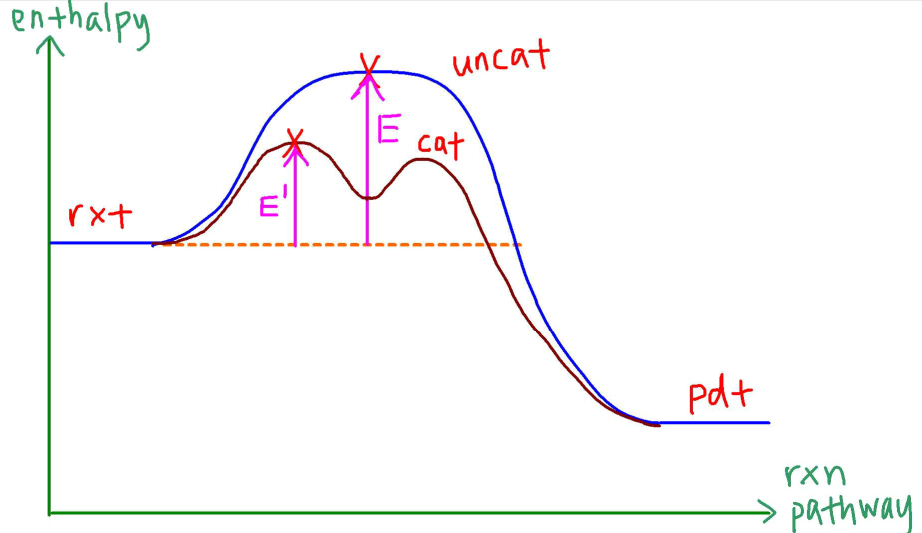
The Fe2+ changes the mechanism since it interferes with the reaction directly.
Hence the number of steps in the mechanism will increase but activation energy is lowered and reaction is faster.
Topic: Transition Elements, Inorganic Chemistry, A Level Chemistry, Singapore
Back to other previous Inorganic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
