Triiodomethane or Iodoform Test
In this video created by Chemistry Guru, Singapore's leading JC Chemistry tuition centre, we want to discuss the triiodomethane test or iodoform test.
The reagents and conditions to administer the test is I2 in NaOH(aq), warm.
The observation for a positive test is the formation of a yellow precipitate.
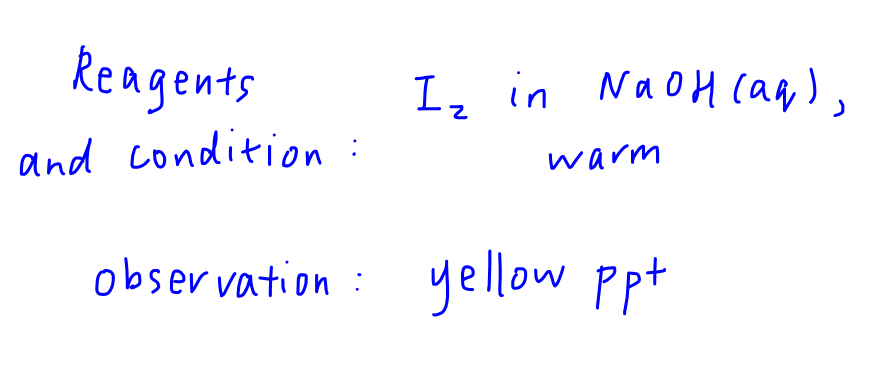
This test is used to identify certain types of alcohols and carbonyl compounds which contain these specific structures:
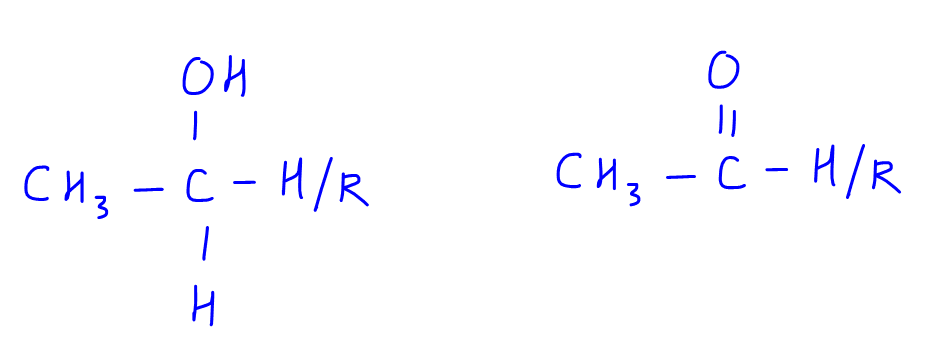
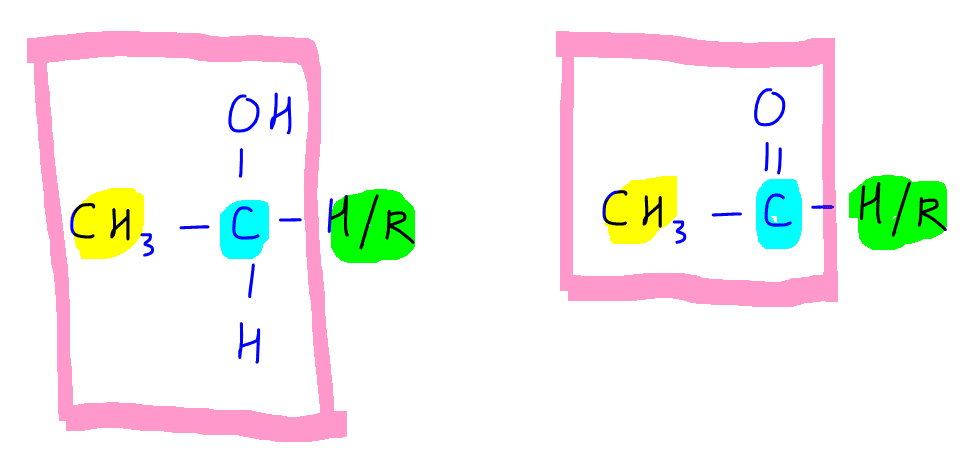
If we study the 2 structures closely the common groups that are required are:
1. Methyl CH3 group (yellow)
2. Carbon that carrys an oxygen, either an alcohol carbon CH(OH) or a carbonyl carbon C=O (blue)
3. Hydrogen or alkyl group (green)
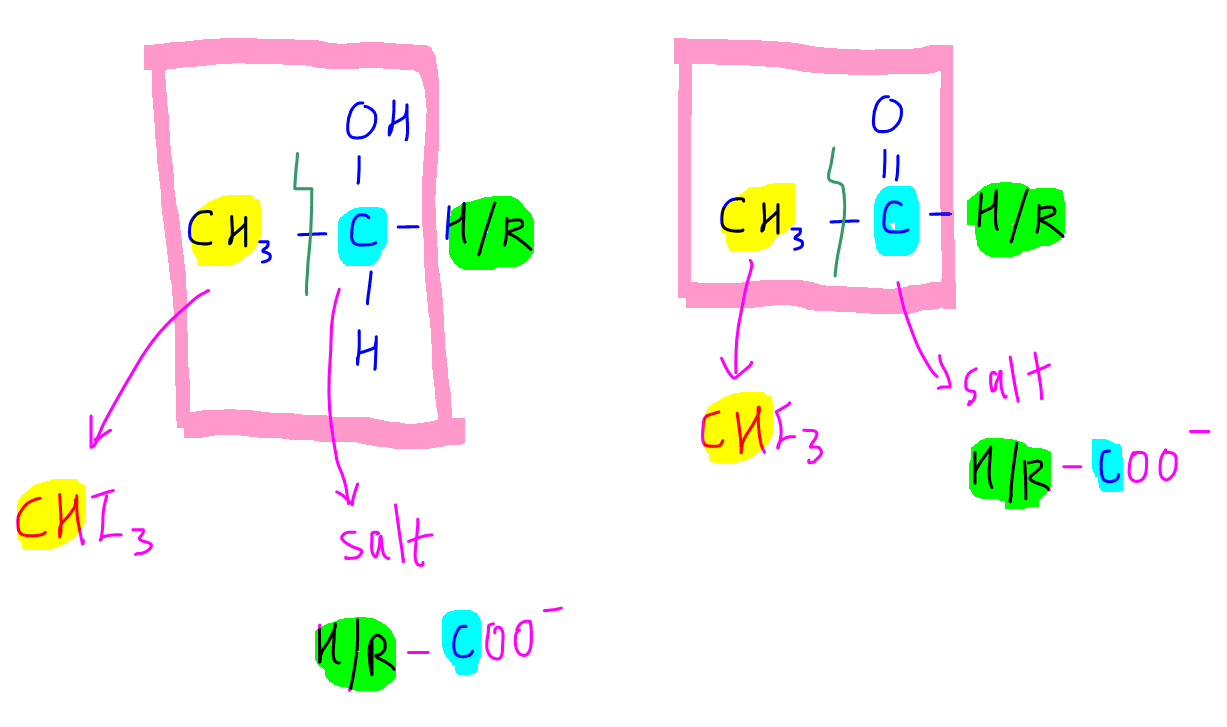
On reaction with iodine in alkaline conditions, the methyl group will be converted to triiodomethane or iodoform which gives us the observation of yellow precipitate.
The carbon which carries the oxygen will be converted to salt of carboxylic acid or conjugate base of carboxylic acid, H/R-COO-
Students will need to identify compounds which have the required structures to give a positive triiodomethane test.
Here are 3 examples for students to try out. Which of these compounds will give a positive triiodomethane test?
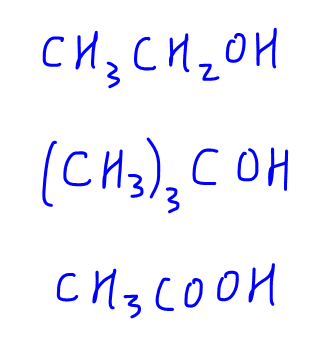
To find out the answers, check out this video now!
Topic: Alcohols, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's reputable A Level Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online chemistry classes!
