Valence Shell Electron Pair Repulsion (VSEPR) Theory and Shapes of Molecules
The Valence Shell Electron Pair Repulsion (VSEPR) Theory can be used to predict the shapes of molecules based on the number of electron pairs around the central atom.
Therefore we need to be familiar with drawing dot-and-cross diagrams for simple molecules first before we can apply VSEPR Theory correctly.
1. Basic Shape
As electron pairs are negatively charged and will repel each other, the electron pairs around the central atom will adopt a shape that will minimise repulsion between them.
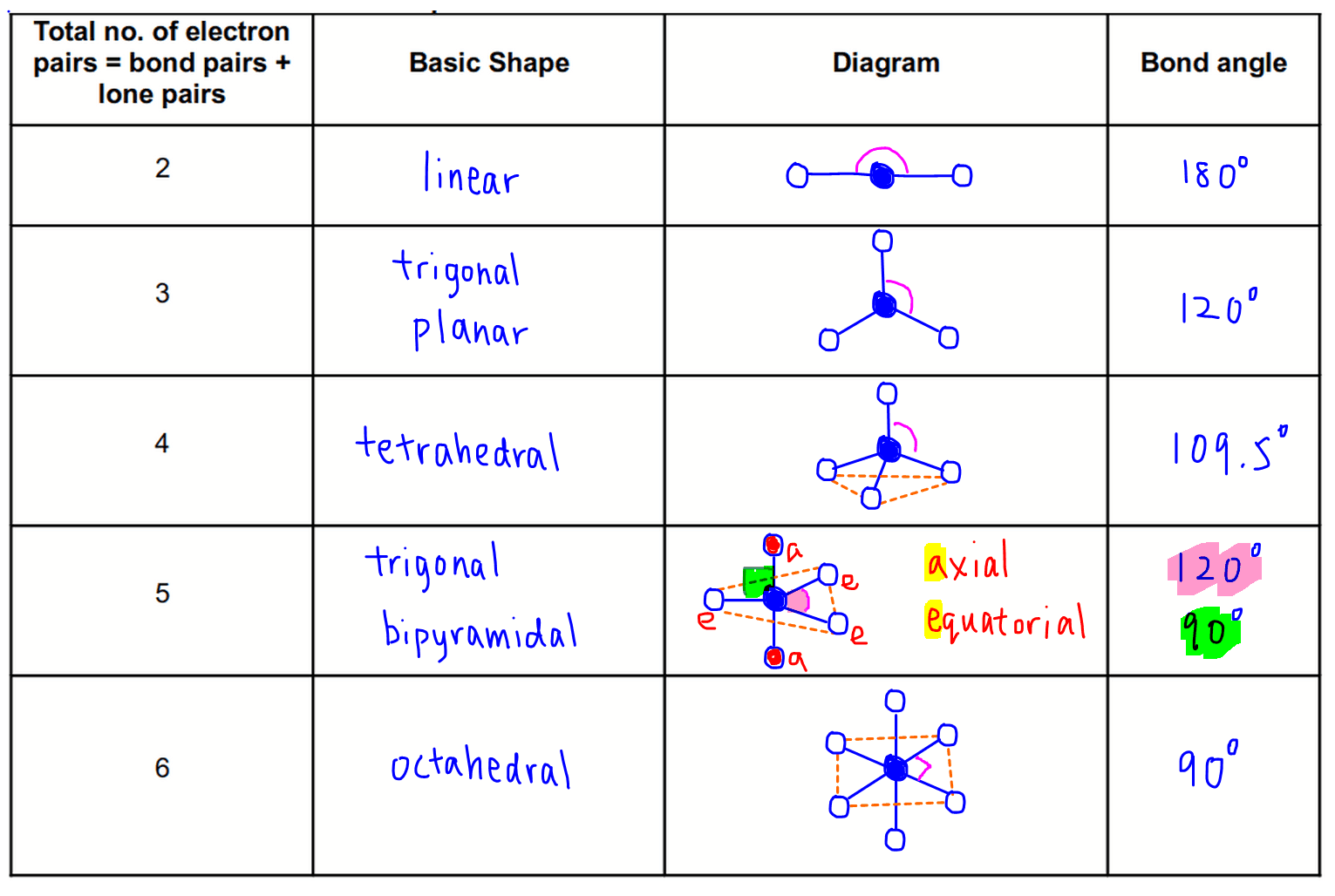
So based on the total number of electron pairs, there are a few distinct basic shapes and bond angles that we need memorise.
2 electron pairs - linear
3 electron pairs - trigonal planar
4 electron pairs - tetrahedral
5 electron pairs - trigonal bipyramidal
6 electron pairs - octahedral
2. Actual Shape
The actual shape depends on the number of bond pairs and lone pairs around the central atom.
In VSEPR Theory, single bond, double bond and triple bond are all treated as one bond pair each.
When we name the shape of an actual molecule, we do not take the lone pair into consideration but the lone pair still exerts a repulsion and affects the shape of the molecule.
Also, the repulsion involving a lone pair is greater than repulsion involving a bond pair, hence the presence of a lone pair will decrease the bond angles as it will squeeze the bond pairs closer together.
An estimate that is useful to determine the bond angle without memorising is for every lone pair, the bond angle will decrease by 2 degree.
2 electron pairs
2 bond pairs + 0 lone pair = linear
3 electron pairs
3 bond pairs + 0 lone pair = trigonal planar
2 bond pairs + 1 lone pair = bent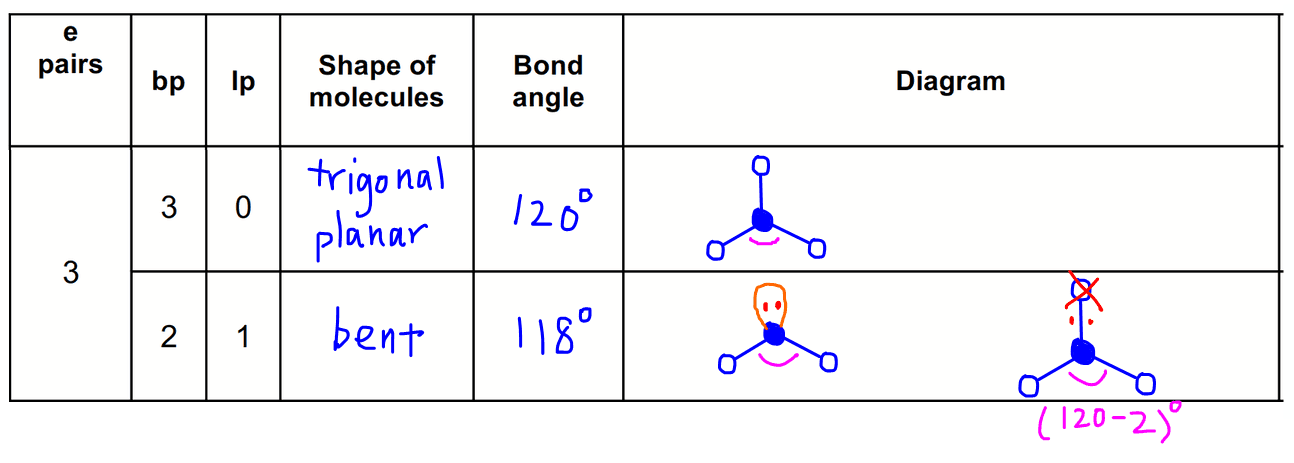
4 electron pairs
4 bond pairs + 0 lone pair = tetrahedral
3 bond pairs + 1 lone pair = trigonal pyramidal
2 bond pairs + 2 lone pairs = bent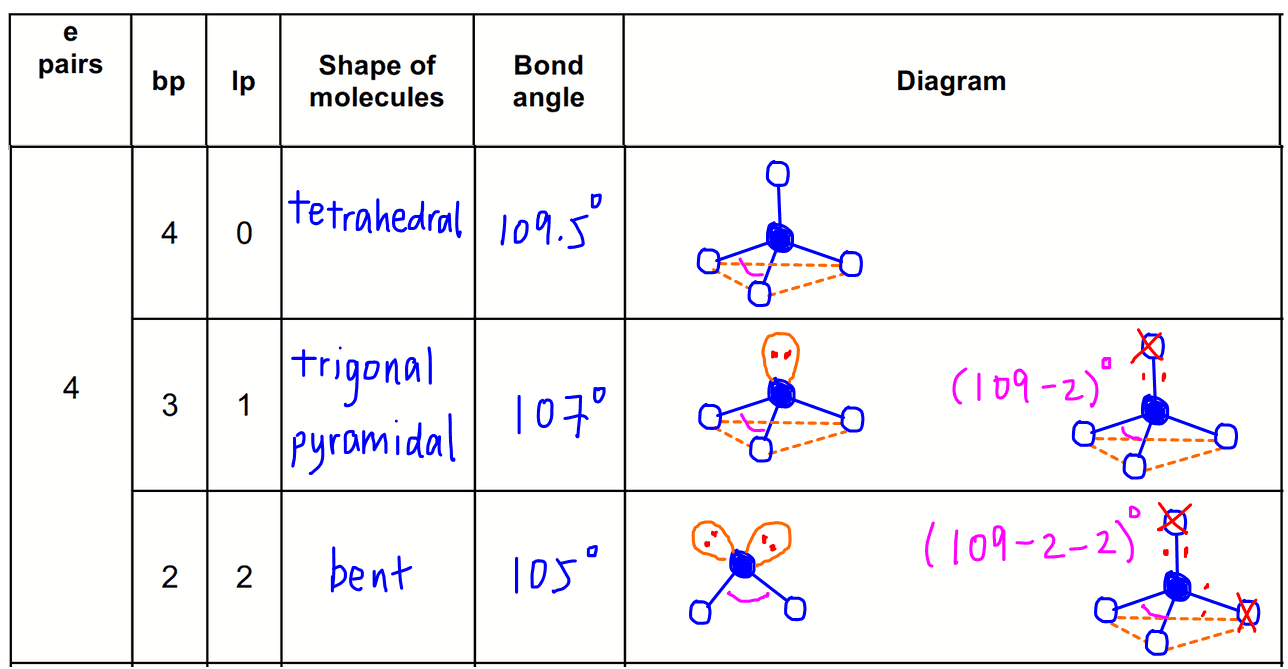
5 electron pairs
5 bond pairs + 0 lone pair = trigonal bipyramidal
4 bond pairs + 1 lone pair = see-saw
3 bond pairs + 2 lone pairs = T-shape
2 bond pairs + 3 lone pairs = linear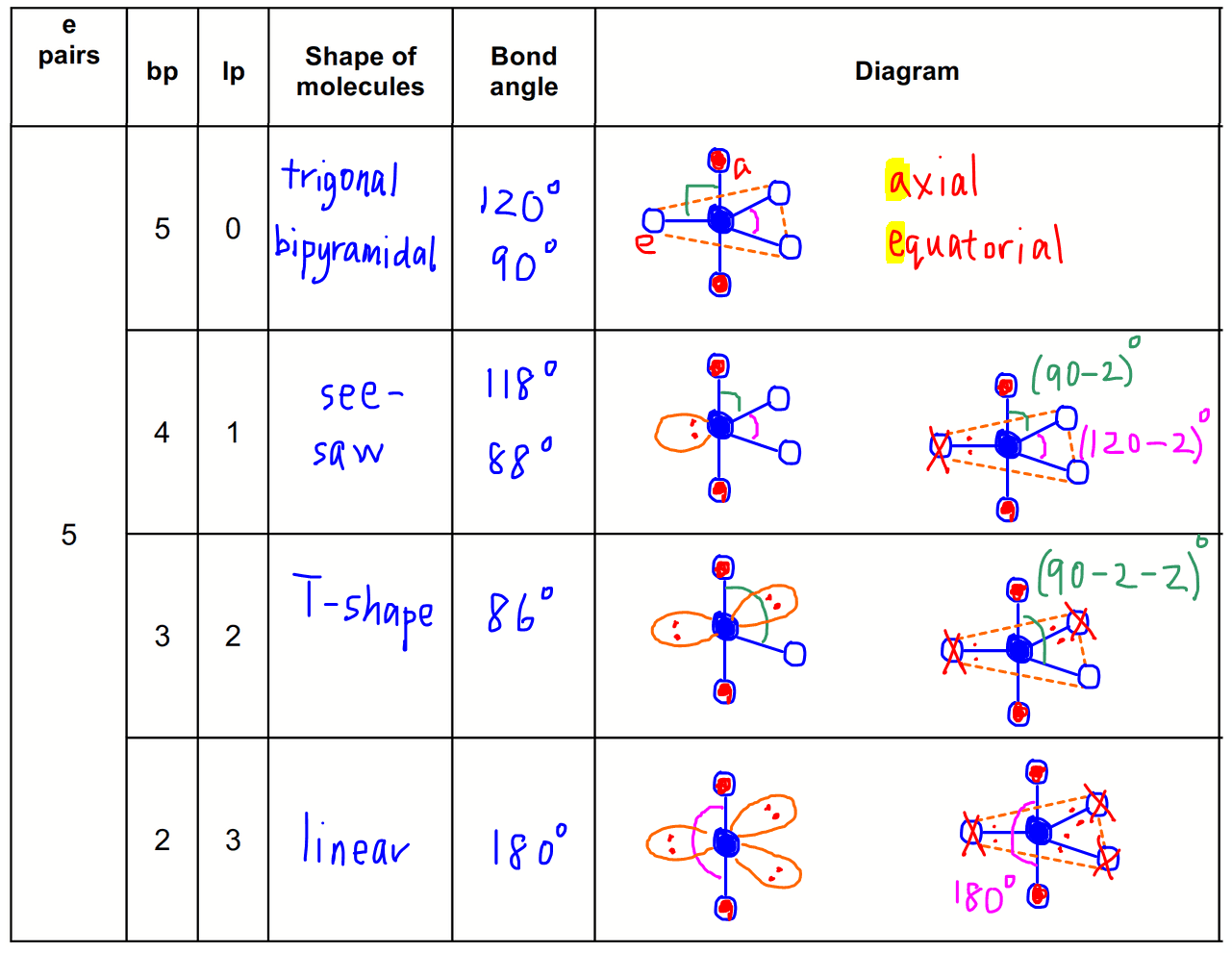
6 electron pairs
6 bond pairs + 0 lone pair = octahedral
5 bond pairs + 1 lone pair = square pyramidal
4 bond pairs + 2 lone pairs = square planar
3 bond pairs + 3 lone pairs = T-shape
2 bond pairs + 4 lone pairs = linear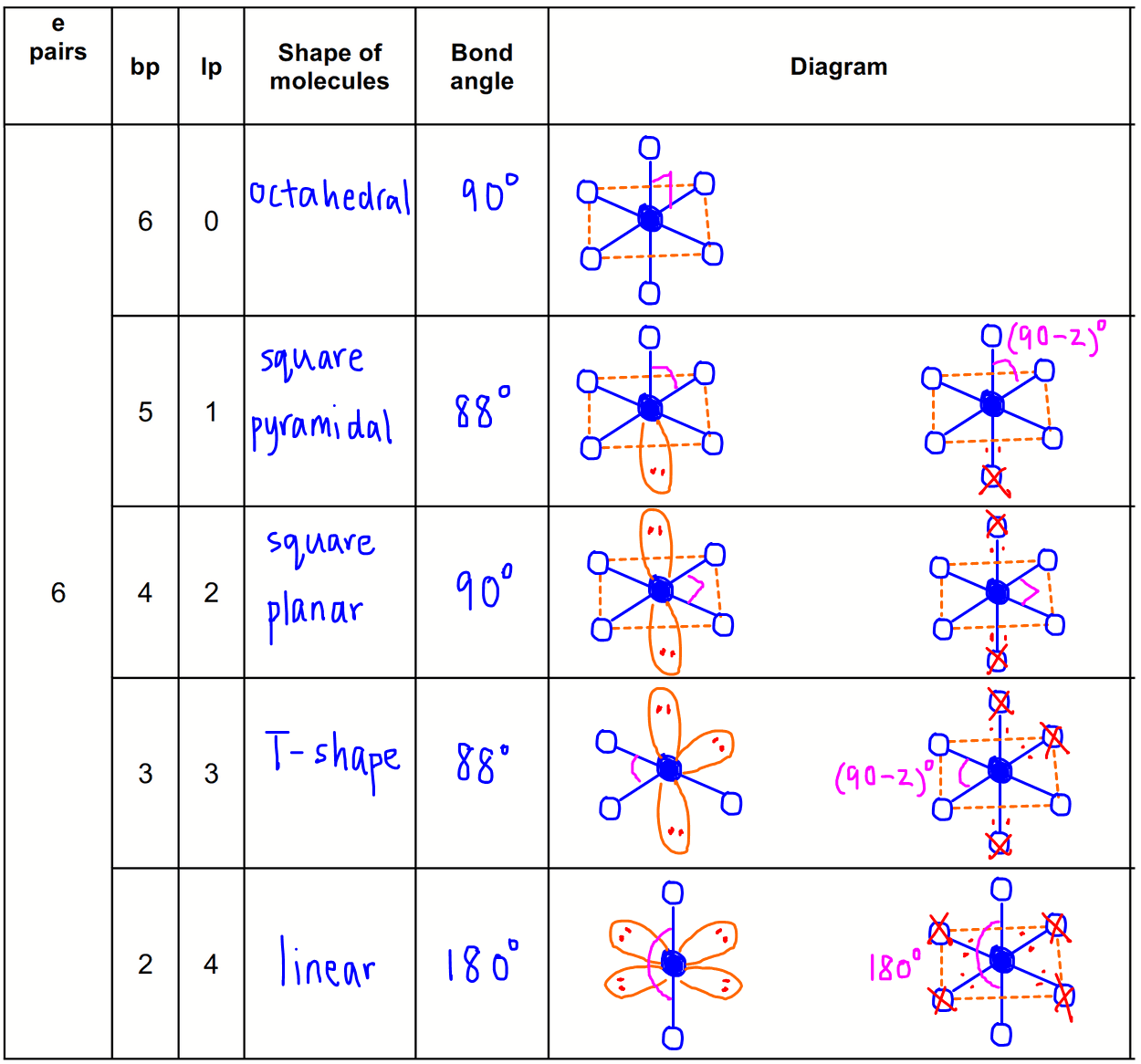
For the detailed step-by-step discussion on how to determine shapes of molecules using VSEPR Theory, check out this video!
Topic: Chemical Bonding, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's reputable A Level Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online tuition classes!
