Determine pH of water at 70 degree celsius
Let Chemistry Guru, Singapore's esteemed A Level Chemistry tuition centre, guide you through our discussion question this week!
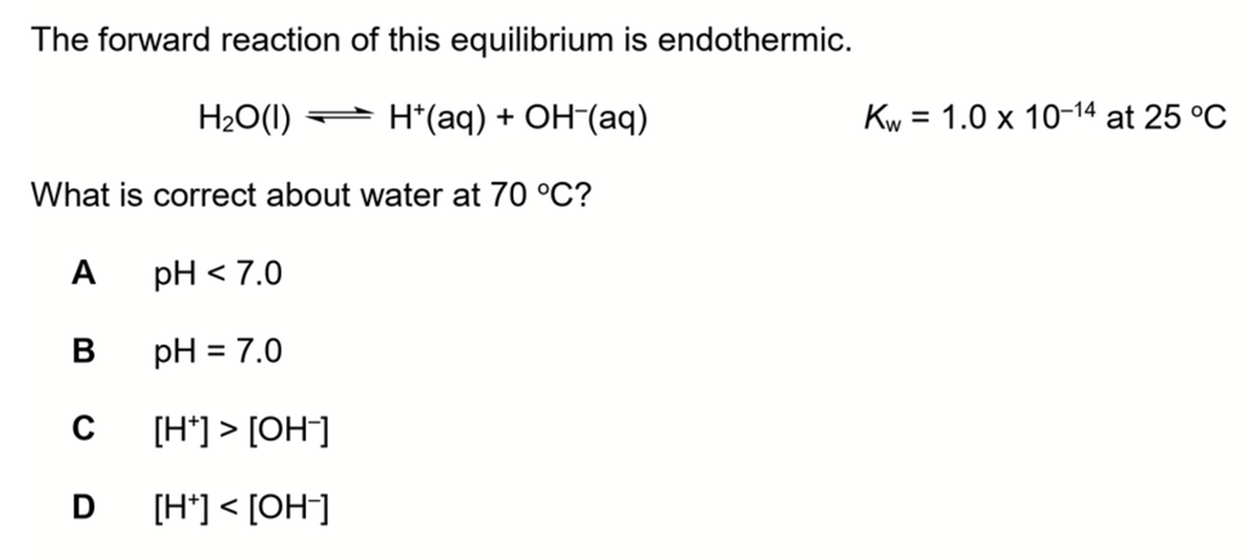
We want to determine which of the above statements about water at 70 degree celsius is correct.
The dissociation in this question is also known as the auto-ionisation of water to give H+ and OH-.
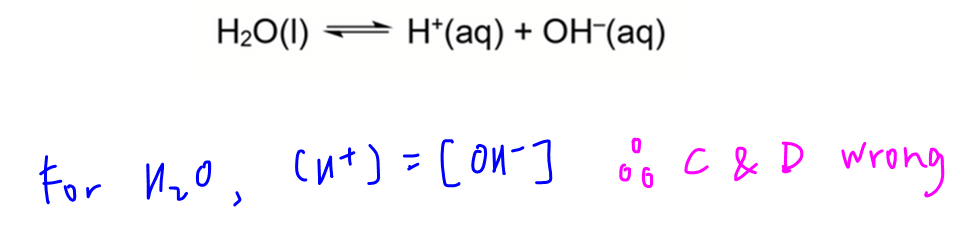
Since 1 water will dissociate to give 1 H+ and 1 OH-, the concentration of H+ and OH- must remain the same.
Hence options C and D are wrong.
We can calculate the pH of water at 25 degree celsius since ionic product of water, Kw, is given.
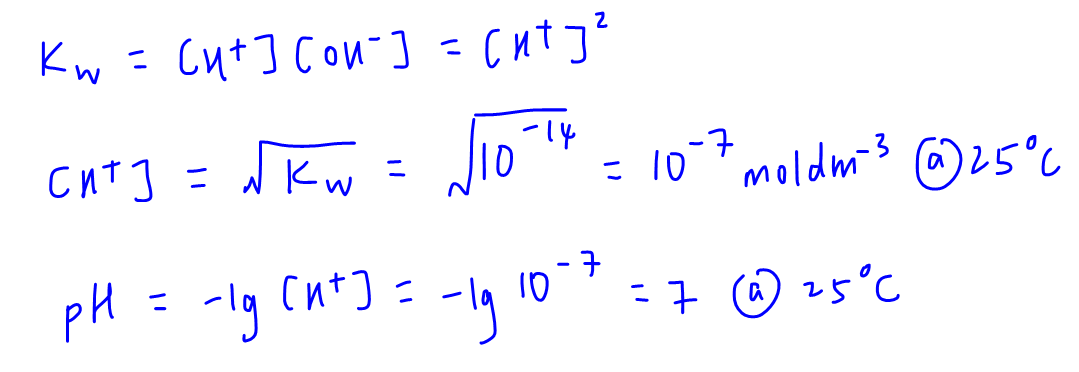
pH of water is found to be 7, which is hardly surprising since we associate pH 7 to be neutral.
But we need to remember that pH of water is calculated to be 7 only when Kw is equal to 10-14.
When temperature changes, Kw will change and therefore pH of water must change.
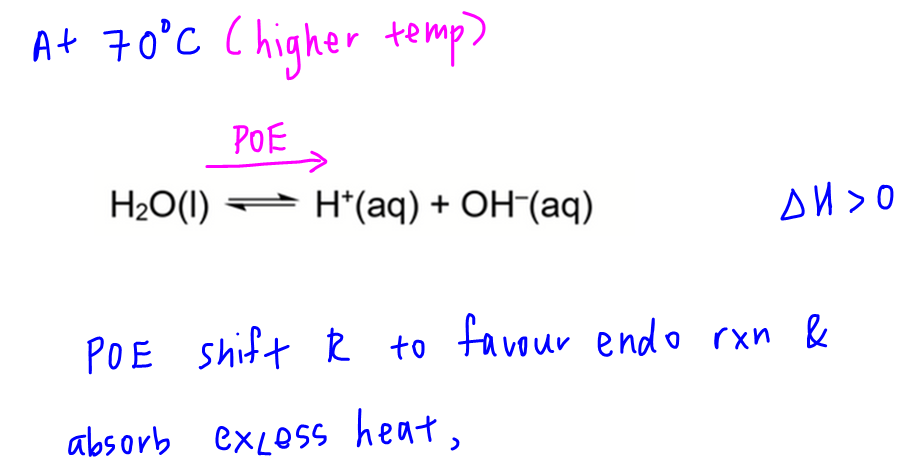
At 70 degree celsius, position of equilibrium shifts to the right favouring the endothermic process and absorb excess heat.
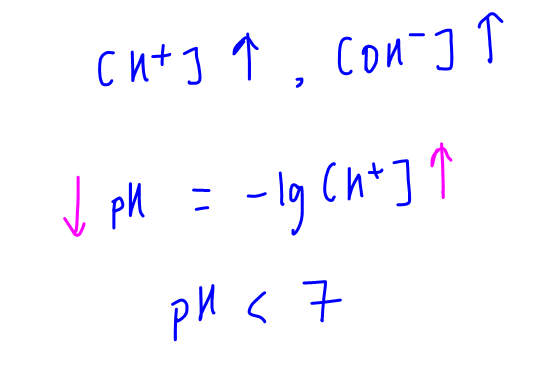
Concentration of H+ and OH- increase and we can deduce pH of water will be less than 7 at higher temperatures.
Some students might wrongly conclude that water must be acidic at 70 degree celsius since pH is less than 7.
This is false since there is also a proportionate increase in the concentration of OH- and the concentration of H+ and OH- remain the same.

Hence water is still neutral at any temperature, regardless of its calculated pH.
Therefore the answer to this question will be option A.

Topic: Ionic Equilibria, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
