What is Oxidation State?
In this JC1 webinar we want to understand what oxidation state is.
Oxidation state of an atom is the charge that it carries as a monatomic ion for ionic compounds, or the hypothetical charge it would have if it were an ion for covalent molecules.
Ionic Compounds
The oxidation state of the element will be its charge as an ion.
For instance in NaCl, charge of sodium ion is +1 hence oxidation state is +1; charge of chloride ion is -1 hence oxidation state is -1.

Covalent Compounds
The oxidation state of the element will be the hypothetical charge if it were an ion.
In this case we have to consider the breaking of all covalent bonds and assign the shared electrons to the more electronegative atom for each covalent bond broken.
We can use this Periodic Table to help compare electronegativity.
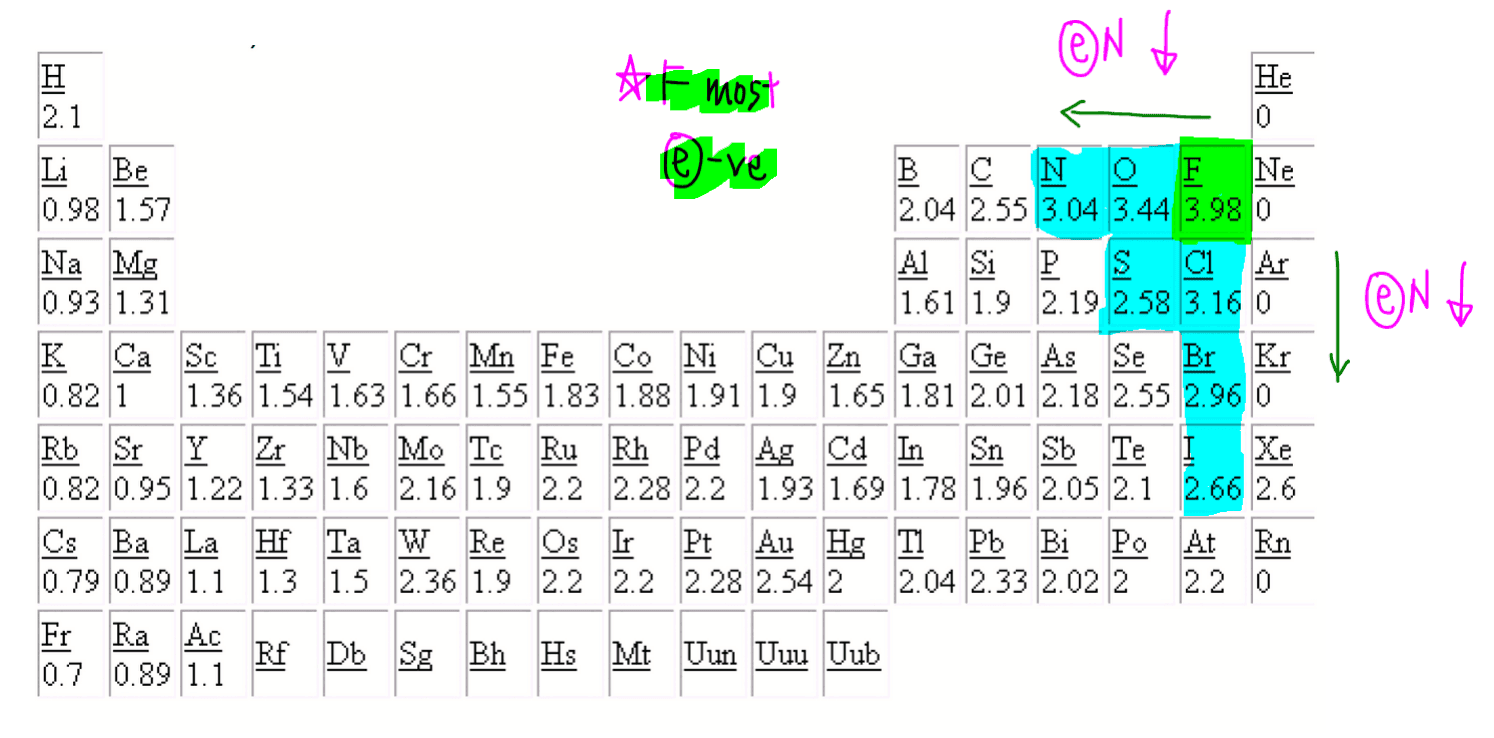
Fluorine is the most electronegative element and electronegativity decreases as we move away from fluorine.
Let's have a few examples to deduce oxidation state of elements in covalent compounds.
Hydrogen Chloride - HCl

Cl is more electronegative than H and will pull the electron pair closer to itself in the covalent bond.
When the H-Cl bond is broken both electrons will go to Cl, hence Cl will gain a -1 charge as an ion.
Therefore oxidation state of Cl in HCl is -1.
H loses its electron hence will gain a +1 charge and oxidation state.
Methane - CH4

C is more electronegative than H hence when C-H bond is broken, C will gain -1 charge and H gains +1 charge.
When all the four C-H bonds are broken, C will gain a total charge and oxidation state of -4; H will have charge and oxidation state of +1.
Chlorine element - Cl2
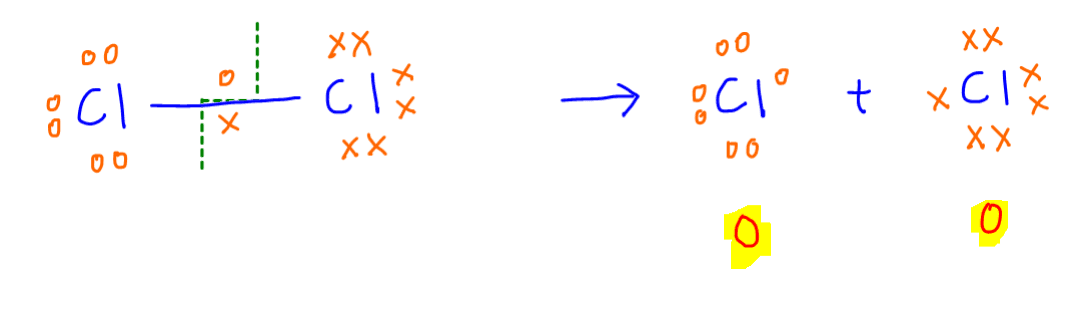
There is no difference in electronegativity in Cl-Cl bond, hence it will break equally to give Cl atoms with zero charge.
Therefore the oxidation state of Cl in Cl2 element is zero.
This is consistent with what we memorised at secondary level where elements have zero oxidation states.
Topic: Redox Reactions, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's trusted JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
