Why Carboxylic Acids undergo Nucleophilic Substitution
In this JC2 webinar we want to understand why carboxylic acids and derivatives undergo nucleophilic substitution instead of addition.
We can deduce the mechanism of a functional group based on the charge and degree of saturation of carbon.
1. Charge of Functional Group Carbon
Positive or partial positive carbon will react with electron rich nucleophiles.
Examples include halogenoalkanes, alcohols, carbonyl compounds, acids and derivatives, and nitrogen compounds where carbon is attached to more electronegative atoms such as halogen, oxygen or nitrogen.
Negative or electron rich carbon will react with electron deficient electrophiles.
Examples include alkenes and arenes.
Neutral or non-polar carbon will only react with free radicals hence they are generally unreactive.
Example will be alkanes.
2. Degree of Saturation of Functional Group Carbon
Saturated or sp3 hybridised carbon will tend to undergo substitution.
Examples are alkanes, halogenoalkanes, alcohols and amines.
Unsaturated or sp2 hybridised carbon will tend to undergo addition.
Examples are alkenes and carbonyl compounds.
We do have exceptions for this in benzene and acids and derivatives, where the carbon is unsaturated but prefers substitution.
Benzene prefers substitution to addition as it wants to retain its resonance stability.
Addition will mean that all carbons in benzene will be saturated, and there will be no more delocalised pi electrons to stabilise benzene.
Therefore substitution is preferred for benzene.
Let's consider nucleophilic substitution of acids and derivatives in detail.

The reason why acids prefer substitution lies in the stability of the intermediate formed.
Let Chemistry Guru, Singapore's renowned JC Chemistry tuition centre, guide you through the mechanism.

In the first step the nucleophile attacks acyl carbon.
The pi bond between carbon and oxygen opens up and both electrons go to oxygen.
This causes the C=O to become C-O, and oxygen to be negatively charged.
Notice this first step is exactly the same as Step 2 in the nucleophilic addition mechanism of carbonyl compounds.
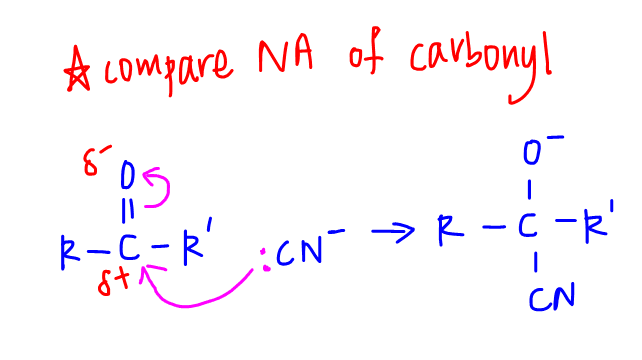
Check out the following video lesson for a more detailed discussion of nucleophilic addition of carbonyl compounds.
So it is still true that since both reactive carbons for acids and carbonyl compounds are partial positive and unsaturated, they both undergo nucleophilic addition.
The difference lies in the stability of the intermediates formed.
For carbonyl compound, intermediate formed has a saturated carbon which is bonded to only 2 electronegative species (O and CN).
Carbon is relatively stable hence will remain saturated, ie addition reaction.
For acid, intermediate formed has a saturated carbon bonded to 3 electronegative species (O, Y and Nu).
Carbon is very positively charged and will eliminate one electronegative Y group to increase stability, ie substitution reaction.
Drawing nucleophilic substitution mechanism for acids and derivatives is not in A Level syllabus but it's useful for understanding organic chem mechanism in general.
Topic: Acids and Derivatives, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's trusted JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
