Electronic Configuration for First 30 Elements
In this video created by Chemistry Guru, Singapore's leading JC Chemistry tuition centre, we want to learn how to write out the electronic configuration for the first 30 elements using the Quantum Model of Atoms.
Based on the Quantum Model, we know that within each principal quantum shell there are subshells and the number of subshells is related to the principal quantum number.
For principal quantum number n = 1, there is 1 subshell 1s.
For principal quantum number n = 2, there are 2 subshells 2s and 2p.
For principal quantum number n = 3, there are 3 subshells 3s, 3p and 3d.
And so on.
Each subshell has a certain number of orbitals and each orbital can hold up to 2 electrons.
Therefore the maximum number of electrons in a subshell is as shown:

When we fill up the electrons, electrons will be added to a subshell with the lowest energy state first, followed by the next most stable state, and so on.
The order of filling up electrons for an element X is as shown:
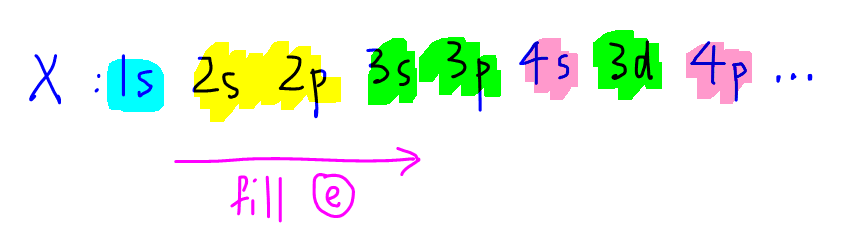
Notice we fill up electrons to 4s subshell first followed by 3d subshell as 4s subshell is more stable when empty.
When the first 36 electrons are filled, the electronic configuration for element X would look like this:
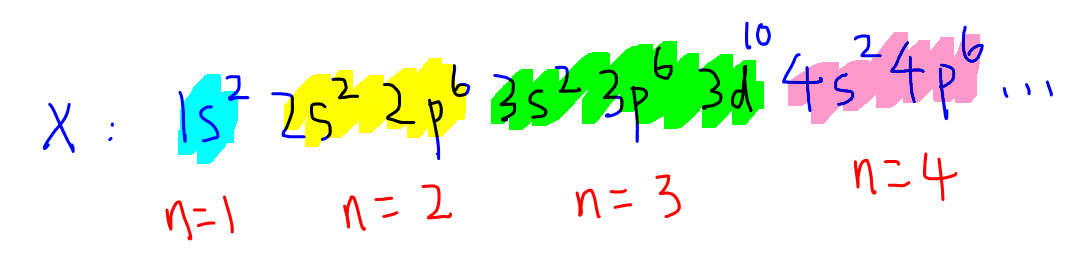
Take note when the electrons are filled, 3d subshell becomes more stable than 4s subshell, hence we write 3d first followed by 4s.
There's a trick to write out the electronic configuration quickly.
Notice that 1s2 2s2 2p6 will add up to 10 electrons.
3s2 3p6 and 4s2 will also add up to 10 electrons.
3d subshell can also hold up to 10 electrons.
So we can add the electrons by sets of 10 to quickly determine the electronic configuration.
In this video we also discuss some examples, namely:
11Na, 26Fe, 26Fe2+, 24Cr and 29Cu
Try writing out the electronic configuration for these examples and check out the video for the solutions!
Topic: Atomic Structure, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's reputable A Level Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online chemistry classes!
