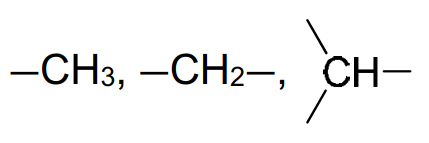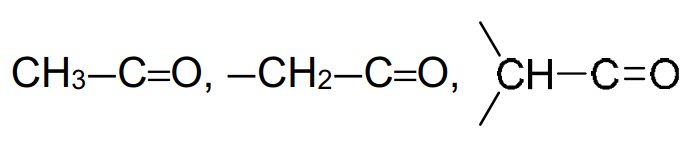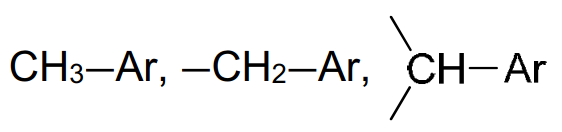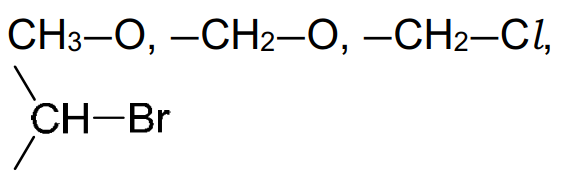Data Booklet for Singapore A Level Chemistry (H1, H2, H3)
Disclaimer: This is the web version of the Data Booklet for Singapore–Cambridge GCE Advanced Level Chemistry (H1, H2 and H3), painstakingly reproduced by Chemistry Guru, Singapore's most reputable JC chemistry tuition centre. Minor adjustments are made for alignment and easier web navigation.
For use in all papers, except practical examinations, for the H1, H2 and H3 syllabuses.
Contents: Tables of Chemical data
1. Important values, constants and standards
2. Ionisation energies (1st, 2nd, 3rd and 4th) of selected elements in kJ mol–1
4. Standard electrode potential and redox potentials, Eθ at 298 K (25 oC)
6. Typical proton (1H) chemical shift values (δ) relative to TMS = 0
7. Characteristic infra-red absorption frequencies for some selected bonds
8. The orientating effect of groups in aromatic substitution reactions
10. The Periodic Table of Elements
1. Important values, constants and standards
|
molar gas constant |
R |
= 8.31 J K–1 mol–1 |
|
the Faraday constant |
F |
= 9.65 x104 C mol–1 |
|
the Avogadro constant |
L |
= 6.02 x1023 mol–1 |
|
the Planck constant |
h |
= 6.63 x10–34 J s |
|
speed of light in a vacuum |
c |
= 3.00 x108 m s–1 |
|
rest mass of proton, 11H |
mp = 1.67 x10–27 kg |
|
|
rest mass of neutron, 10n |
mn = 1.67 x10–27 kg |
|
|
rest mass of electron, 0–1 e |
me = 9.11 x 10–31 kg |
|
|
elementary charge |
e |
= 1.60 x10–19 C |
|
molar volume of gas |
Vm = 22.7 dm3 mol–1 at s.t.p. Vm = 24 dm3 mol–1 at r.t.p. (where s.t.p. is expressed as 105 Pa [1 bar] and 273 K [0 oC], r.t.p. is expressed as 101325 Pa [1 atm] and 293 K [20 oC]) |
|
|
ionic product of water |
Kw = 1.00 x10–14 mol2 dm–6 (at 298 K [25 oC]) |
|
|
= 4.18 kJ kg–1 K–1 (= 4.18 J g–1 K–1) |
||
2. Ionisation energies (1st, 2nd, 3rd and 4th) of selected elements, in kJ mol–1
|
|
Proton Number |
First |
Second |
Third |
Fourth |
|
H |
1 |
1310 |
– |
– |
– |
|
He |
2 |
2370 |
5250 |
– |
– |
|
Li |
3 |
519 |
7300 |
11800 |
– |
|
Be |
4 |
900 |
1760 |
14800 |
21000 |
|
B |
5 |
799 |
2420 |
3660 |
25000 |
|
C |
6 |
1090 |
2350 |
4610 |
6220 |
|
N |
7 |
1400 |
2860 |
4590 |
7480 |
|
O |
8 |
1310 |
3390 |
5320 |
7450 |
|
F |
9 |
1680 |
3370 |
6040 |
8410 |
|
Ne |
10 |
2080 |
3950 |
6150 |
9290 |
|
Na |
11 |
494 |
4560 |
6940 |
9540 |
|
Mg |
12 |
736 |
1450 |
7740 |
10500 |
|
Al |
13 |
577 |
1820 |
2740 |
11600 |
|
Si |
14 |
786 |
1580 |
3230 |
4360 |
|
P |
15 |
1060 |
1900 |
2920 |
4960 |
|
S |
16 |
1000 |
2260 |
3390 |
4540 |
|
Cl |
17 |
1260 |
2300 |
3850 |
5150 |
|
Ar |
18 |
1520 |
2660 |
3950 |
5770 |
|
K |
19 |
418 |
3070 |
4600 |
5860 |
|
Ca |
20 |
590 |
1150 |
4940 |
6480 |
|
Sc |
21 |
632 |
1240 |
2390 |
7110 |
|
Ti |
22 |
661 |
1310 |
2720 |
4170 |
|
V |
23 |
648 |
1370 |
2870 |
4600 |
|
Cr |
24 |
653 |
1590 |
2990 |
4770 |
|
Mn |
25 |
716 |
1510 |
3250 |
5190 |
|
Fe |
26 |
762 |
1560 |
2960 |
5400 |
|
Co |
27 |
757 |
1640 |
3230 |
5100 |
|
Ni |
28 |
736 |
1750 |
3390 |
5400 |
|
|
Proton Number |
First |
Second |
Third |
Fourth |
|
Cu |
29 |
745 |
1960 |
3350 |
5690 |
|
Zn |
30 |
908 |
1730 |
3828 |
5980 |
|
Ga |
31 |
577 |
1980 |
2960 |
6190 |
|
Ge |
32 |
762 |
1540 |
3300 |
4390 |
|
Br |
35 |
1140 |
2080 |
3460 |
4850 |
|
Rb |
37 |
403 |
2632 |
3900 |
5080 |
|
Sr |
38 |
548 |
1060 |
4120 |
5440 |
|
Ag |
47 |
731 |
2074 |
3361 |
– |
|
Sn |
50 |
707 |
1410 |
2940 |
3930 |
|
I |
53 |
1010 |
1840 |
3200 |
4030 |
|
Cs |
55 |
376 |
2420 |
3300 |
– |
|
Ba |
56 |
502 |
966 |
3390 |
– |
|
Pb |
82 |
716 |
1450 |
3080 |
3. Bond energies
3(a) Bond energies in diatomic molecules (these are exact values)
Homonuclear
|
Bond |
Energy/kJ mol–1 |
|
H―H |
436 |
|
D―D |
442 |
|
N≡N |
944 |
|
O=O |
496 |
|
F―F |
158 |
|
Cl―Cl |
244 |
|
Br―Br |
193 |
|
I―I |
151 |
Heteronuclear
|
Bond |
Energy/kJ mol–1 |
|
H―F |
562 |
|
H―Cl |
431 |
|
H―Br |
366 |
|
H―I |
299 |
|
C≡O |
1077 |
3(b) Bond energies in polyatomic molecules (these are average values)
Homonuclear
|
Bond |
Energy/kJ mol–1 |
|
C―C |
350 |
|
C=C |
610 |
|
C≡C |
840 |
|
C=C (benzene) |
520 |
|
N―N |
160 |
|
N=N |
410 |
|
O―O |
150 |
|
Si―Si |
222 |
|
P―P |
200 |
|
S―S |
264 |
Heteronuclear
|
Bond |
Energy/kJ mol–1 |
|
C―H |
410 |
|
C―F |
485 |
|
C―Cl |
340 |
|
C―Br |
280 |
|
C―I |
240 |
|
C―N |
305 |
|
C=N |
610 |
|
C≡N |
890 |
|
C―O |
360 |
|
C=O |
740 |
|
C=O in CO2 |
805 |
|
N―H |
390 |
|
N―Cl |
310 |
|
O―H |
460 |
|
Si―Cl |
359 |
|
Si―H |
320 |
|
Si―O (in SiO2(s)) |
460 |
|
Si=O (in SiO2(g)) |
640 |
|
P―H |
320 |
|
P―Cl |
330 |
|
P―O |
340 |
|
P=O |
540 |
|
S―H |
347 |
|
S―Cl |
250 |
|
S―O |
360 |
|
S=O |
4. Standard electrode potential and redox potentials, Eθ at 298 K (25oC)
For ease of reference, two tabulations are given:
(a) an extended list in alphabetical order;
(b) a shorter list in decreasing order of magnitude, i.e. a redox series.
4(a) Eθ in alphabetical order
|
Electrode reaction |
Eθ/ V |
||
|
Ag+ + e– |
⇌ |
Ag |
+0.80 |
|
Al3+ + 3e– |
⇌ |
Al |
–1.66 |
|
Ba2+ + 2e– |
⇌ |
Ba |
–2.90 |
|
Br2 + 2e– |
⇌ |
2Br– |
+1.07 |
|
Ca2+ + 2e– |
⇌ |
Ca |
–2.87 |
|
Cl2 + 2e– |
⇌ |
2Cl– |
+1.36 |
|
2HOCl + 2H+ + 2e– |
⇌ |
Cl2 + 2H2O |
+1.64 |
|
ClO– + H2O + 2e– |
⇌ |
Cl– + 2OH– |
+0.81 |
|
Co2+ + 2e– |
⇌ |
Co |
–0.28 |
|
Co3+ + e– |
⇌ |
Co2+ |
+1.89 |
|
[Co(NH3)6]2+ + 2e– |
⇌ |
Co + 6NH3 |
–0.43 |
|
Cr2+ + 2e– |
⇌ |
Cr |
–0.91 |
|
Cr3+ + 3e– |
⇌ |
Cr |
–0.74 |
|
Cr3+ + e– |
⇌ |
Cr2+ |
–0.41 |
|
Cr2O72– + 14H+ + 6e– |
⇌ |
2Cr3+ + 7H2O |
+1.33 |
|
Cu+ + e– |
⇌ |
Cu |
+0.52 |
|
Cu2+ + 2e– |
⇌ |
Cu |
+0.34 |
|
Cu2+ + e– |
⇌ |
Cu+ |
+0.15 |
|
[Cu(NH3)4]2+ + 2e– |
⇌ |
Cu + 4NH3 |
–0.05 |
|
F2 + 2e– |
⇌ |
2F– |
+2.87 |
|
Fe2+ + 2e– |
⇌ |
Fe |
–0.44 |
|
Fe3+ + 3e– |
⇌ |
Fe |
–0.04 |
|
Electrode reaction |
Eθ/ V |
||
|
Fe3+ + e– |
⇌ |
Fe2+ |
+0.77 |
|
[Fe(CN)6]3– + e– |
⇌ |
[Fe(CN)6]4– |
+0.36 |
|
Fe(OH)3 + e– |
⇌ |
Fe(OH)2 + OH– |
–0.56 |
|
2H+ + 2e– |
⇌ |
H2 |
0.00 |
|
I2 + 2e– |
⇌ |
2I– |
+0.54 |
|
K+ + e– |
⇌ |
K |
–2.92 |
|
Li+ + e– |
⇌ |
Li |
–3.04 |
|
Mg2+ + 2e– |
⇌ |
Mg |
–2.38 |
|
Mn2+ + 2e– |
⇌ |
Mn |
–1.18 |
|
Mn3+ + e– |
⇌ |
Mn2+ |
+1.54 |
|
MnO2 + 4H+ + 2e– |
⇌ |
Mn2+ + 2H2O |
+1.23 |
|
MnO4– + e– |
⇌ |
MnO42– |
+0.56 |
|
MnO4– + 4H+ + 3e– |
⇌ |
MnO2 + 2H2O |
+1.67 |
|
MnO4– + 8H+ + 5e– |
⇌ |
Mn2+ + 4H2O |
+1.52 |
|
NO3– + 2H+ + e– |
⇌ |
NO2 + H2O |
+0.81 |
|
NO3– + 3H+ + 2e– |
⇌ |
HNO2 + H2O |
+0.94 |
|
NO3– + 10H+ + 8e– |
⇌ |
NH4+ + 3H2O |
+0.87 |
|
Na+ + e– |
⇌ |
Na |
–2.71 |
|
Ni2+ + 2e– |
⇌ |
Ni |
–0.25 |
|
[Ni(NH3)6]2+ + 2e– |
⇌ |
Ni + 6NH3 |
–0.51 |
|
H2O2 + 2H+ + 2e– |
⇌ |
2H2O |
+1.77 |
|
HO2– + H2O + 2e– |
⇌ |
3OH– |
+0.88 |
|
O2 + 4H+ + 4e– |
⇌ |
2H2O |
+1.23 |
|
O2 + 2H2O + 4e– |
⇌ |
4OH– |
+0.40 |
|
O2 + 2H+ + 2e– |
⇌ |
H2O2 |
+0.68 |
|
Electrode reaction |
Eθ/ V |
||
|
O2 + H2O + 2e– |
⇌ |
HO2– + OH– |
–0.08 |
|
2H2O + 2e– |
⇌ |
H2 + 2OH– |
–0.83 |
|
Pb2+ + 2e– |
⇌ |
Pb |
–0.13 |
|
Pb4+ + 2e– |
⇌ |
Pb2+ |
+1.69 |
|
PbO2 + 4H+ + 2e– |
⇌ |
Pb2+ + 2H2O |
+1.47 |
|
SO42– + 4H+ + 2e– |
⇌ |
SO2 + 2H2O |
+0.17 |
|
S2O82– + 2e– |
⇌ |
2SO42– |
+2.01 |
|
S4O62– + 2e– |
⇌ |
2S2O32– |
+0.09 |
|
Sn2+ + 2e– |
⇌ |
Sn |
–0.14 |
|
Sn4+ + 2e– |
⇌ |
Sn2+ |
+0.15 |
|
V2+ + 2e– |
⇌ |
V |
–1.20 |
|
V3+ + e– |
⇌ |
V2+ |
–0.26 |
|
VO2+ + 2H+ + e– |
⇌ |
V3+ + H2O |
+0.34 |
|
VO2+ + 2H+ + e– |
⇌ |
VO2+ + H2O |
+1.00 |
|
VO3– + 4H+ + e– |
⇌ |
VO2+ + 2H2O |
+1.00 |
|
Zn2+ + 2e– |
⇌ |
Zn |
–0.76 |
All ionic states refer to aqueous ions but other state symbols have been omitted.
4(b) Eθ in decreasing order of oxidising power
(a selection only – see also the extended alphabetical list on the previous pages)
|
Electrode reaction |
Eθ/ V |
||
|
F2 + 2e– |
⇌ |
2F– |
+2.87 |
|
S2O82– + 2e– |
⇌ |
2SO42– |
+2.01 |
|
H2O2 + 2H+ + 2e– |
⇌ |
2H2O |
+1.77 |
|
MnO4– + 8H+ + 5e– |
⇌ |
Mn2+ + 4H2O |
+1.52 |
|
PbO2 + 4H+ + 2e– |
⇌ |
Pb2+ + 2H2O |
+1.47 |
|
Cl2 + 2e– |
⇌ |
2Cl– |
+1.36 |
|
Cr2O72– + 14H+ + 6e– |
⇌ |
2Cr3+ + 7H2O |
+1.33 |
|
O2 + 4H+ + 4e– |
⇌ |
2H2O |
+1.23 |
|
Br2 + 2e– |
⇌ |
2Br– |
+1.07 |
|
NO3– + 10H+ + 8e– |
⇌ |
NH4+ + 3H2O |
+0.87 |
|
ClO– + H2O + 2e– |
⇌ |
Cl– + 2OH– |
+0.81 |
|
NO3– + 2H+ + e– |
⇌ |
NO2 + H2O |
+0.81 |
|
Ag+ + e– |
⇌ |
Ag |
+0.80 |
|
Fe3+ + e– |
⇌ |
Fe2+ |
+0.77 |
|
I2 + 2e– |
⇌ |
2I– |
+0.54 |
|
O2 + 2H2O + 4e– |
⇌ |
4OH– |
+0.40 |
|
Cu2+ + 2e– |
⇌ |
Cu |
+0.34 |
|
SO42– + 4H+ + 2e– |
⇌ |
SO2 + 2H2O |
+0.17 |
|
Sn4+ + 2e– |
⇌ |
Sn2+ |
+0.15 |
|
S4O62– + 2e– |
⇌ |
2S2O32– |
+0.09 |
|
2H+ + 2e– |
⇌ |
H2 |
0.00 |
|
Pb2+ + 2e– |
⇌ |
Pb |
–0.13 |
|
Sn2+ + 2e– |
⇌ |
Sn |
–0.14 |
|
Fe2+ + 2e– |
⇌ |
Fe |
–0.44 |
|
Electrode reaction |
Eθ/ V |
||
|
Zn2+ + 2e– |
⇌ |
Zn |
–0.76 |
|
2H2O + 2e– |
⇌ |
H2 + 2OH– |
–0.83 |
|
V2+ + 2e– |
⇌ |
V |
–1.20 |
|
Mg2+ + 2e– |
⇌ |
Mg |
–2.38 |
|
Ca2+ + 2e– |
⇌ |
Ca |
–2.87 |
|
K+ + e– |
⇌ |
K |
|
5. Atomic and ionic radii
|
(a) Period 1 |
atomic/nm |
ionic/nm |
||||
|
single covalent |
H |
0.037 |
H– |
0.208 |
||
|
van der Waals |
He |
0.140 |
||||
|
(b) Period 2 |
||||||
|
metallic |
Li |
0.152 |
Li+ |
0.060 |
||
|
Be |
0.112 |
Be2+ |
0.031 |
|||
|
single covalent |
B |
0.080 |
B3+ |
0.020 |
||
|
C |
0.077 |
C4+ |
0.015 |
C4– |
0.260 |
|
|
N |
0.074 |
N3– |
0.171 |
|||
|
O |
0.073 |
O2– |
0.140 |
|||
|
F |
0.072 |
F– |
0.136 |
|||
|
van der Waals |
Ne |
0.160 |
||||
|
(c) Period 3 |
||||||
|
metallic |
Na |
0.186 |
Na+ |
0.095 |
||
|
Mg |
0.160 |
Mg2+ |
0.065 |
|||
|
Al |
0.143 |
Al3+ |
0.050 |
|||
|
single covalent |
Si |
0.117 |
Si4+ |
0.041 |
||
|
P |
0.110 |
P3– |
0.212 |
|||
|
S |
0.104 |
S2– |
0.184 |
|||
|
Cl |
0.099 |
Cl– |
0.181 |
|||
|
van der Waals |
Ar |
0.190 |
||||
|
(d) Group 2 |
||||||
|
metallic |
Be |
0.112 |
Be2+ |
0.031 |
||
|
Mg |
0.160 |
Mg2+ |
0.065 |
|||
|
Ca |
0.197 |
Ca2+ |
0.099 |
|||
|
Sr |
0.215 |
Sr2+ |
0.113 |
|||
|
Ba |
0.217 |
Ba2+ |
0.135 |
|||
|
Ra |
0.220 |
Ra2+ |
0.140 |
|||
|
(e) Group 14 |
atomic/nm |
ionic/nm |
||||
|
single covalent |
C |
0.077 |
||||
|
Si |
0.117 |
Si4+ |
0.041 |
|||
|
Ge |
0.122 |
Ge2+ |
0.093 |
|||
|
metallic |
Sn |
0.162 |
Sn2+ |
0.112 |
||
|
Pb |
0.175 |
Pb2+ |
0.120 |
|||
|
(f) Group 17 |
||||||
|
single covalent |
F |
0.072 |
F– |
0.136 |
||
|
Cl |
0.099 |
Cl– |
0.181 |
|||
|
Br |
0.114 |
Br– |
0.195 |
|||
|
I |
0.133 |
I– |
0.216 |
|||
|
At |
0.140 |
|||||
|
(g) First row d block elements |
||||||
|
metallic |
Sc |
0.164 |
Sc3+ |
0.075 |
||
|
Ti |
0.146 |
Ti2+ |
0.086 |
Ti3+ |
0.067 |
|
|
V |
0.135 |
V2+ |
0.079 |
V3+ |
0.064 |
|
|
Cr |
0.129 |
Cr2+ |
0.073 |
Cr3+ |
0.062 |
|
|
Mn |
0.132 |
Mn2+ |
0.083 |
Mn3+ |
0.058 |
|
|
Fe |
0.126 |
Fe2+ |
0.061 |
Fe3+ |
0.055 |
|
|
Co |
0.125 |
Co2+ |
0.065 |
Co3+ |
0.055 |
|
|
Ni |
0.124 |
Ni2+ |
0.069 |
Ni3+ |
0.056 |
|
|
Cu |
0.128 |
Cu2+ |
0.073 |
|||
|
Zn |
0.135 |
Zn2+ |
||||
6. Typical proton (1H) chemical shift values (δ) relative to TMS = 0
|
Type of proton |
Environment of proton |
Example structures |
Chemical Shift range (δ) |
|
C―H |
alkane |
|
0.9–1.7 |
|
alkyl next to C=O |
|
2.2–3.0 |
|
|
alkyl next to aromatic ring |
|
2.3–3.0 |
|
|
alkyl next to electronegative atom |
|
3.2–4.0 |
|
|
attached to alkyne |
≡C―H |
1.8–3.1 |
|
|
attached to alkene |
=CH2, =CH― |
4.5–6.0 |
|
|
attached to aromatic ring |
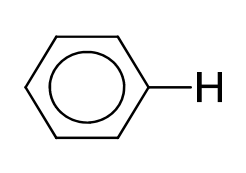
|
6.0–9.0 |
|
|
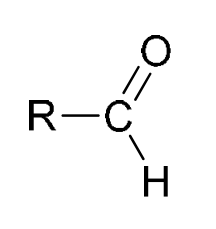
aldehyde |
|
9.3–10.5 |
|
|
O―H (see note below) |
alcohol |
RO―H |
0.5–6.0 |
|
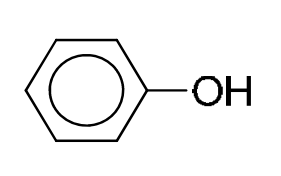
phenol |
|
4.5–7.0 |
|
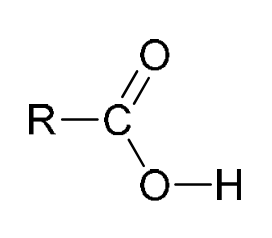
|
carboxylic acid |
|
9.0–13.0 |
|
|
N―H (see note below) |
alkyl amine |
R―NH― |
1.0–5.0 |
|
aryl amine |
|
3.0–6.0 |
|
|
amide |
|
5.0–12.0 |
Note: δvalues for ―O―H and ―N―H protons can vary depending on solvent and concentration.
7. Characteristic infra-red absorption frequencies for some selected bonds
|
Bond |
Functional groups containing the bond |
Absorption range (in wave numbers) / cm–1 |
Appearance of peak (s = strong, w = weak) |
|
C―Cl |
chloroalkanes |
700–800 |
s |
|
C―O |
alcohol |
970–1260 |
s |
|
ether |
1000–1310 |
s |
|
|
ester |
1050–1330 |
s |
|
|
carboxylic acids |
1210–1440 |
s |
|
|
C=C |
aromatic |
1475–1625 |
s |
|
alkenes |
1635–1690 |
w |
|
|
C=O |
amides |
1640–1690 |
s |
|
ketones and aldehydes |
1670–1740 |
s |
|
|
carboxylic acids |
1680–1730 |
s |
|
|
esters |
1710–1750 |
s |
|
|
C≡C |
alkynes |
2150–2250 |
w unless conjugated |
|
C≡N |
nitriles |
2200–2250 |
w |
|
C―H |
alkanes, CH2―H |
2850–2950 |
s |
|
alkenes/arenes, =C―H |
3000–3100 |
w |
|
|
N―H |
amines, amides |
3300–3500 |
w |
|
O―H |
carboxylic acid, RCO2―H |
2500–3000 |
s and very broad |
|
H-bonded alcohol/phenol, RO―H |
3200–3600 |
s |
|
|
free alcohol, RO―H |
3580–3650 |
8. The orientating effect of groups in aromatic substitution reactions
The position of the incoming group, E, is determined by the nature of the group, G, already bonded to the ring, and not by the nature of the incoming group E.
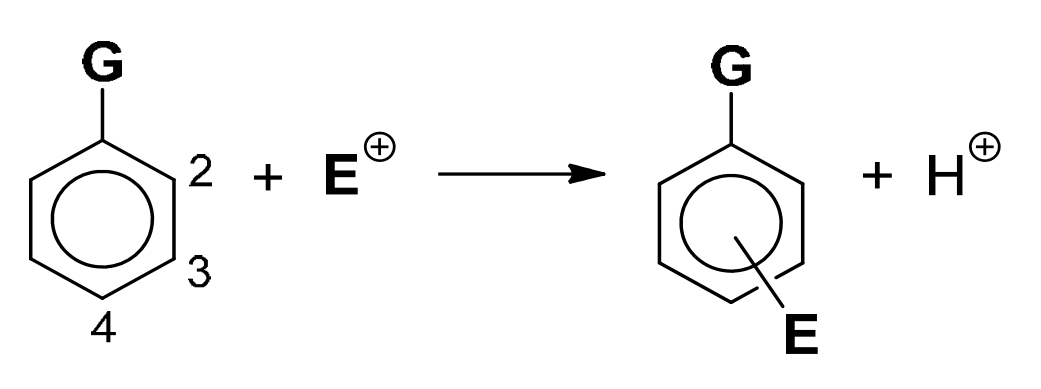
|
G |
―alkyl ―OH or ―OR ―NH2, ―NHR or ―NR2 ―NHCOR |
―Cl, ―Br, ―I |
―CHO, ―COR ―CO2H, ―CO2R ―NH3+ ―NO2, ―CN |
|
Reactivity of ring (compared to benzene) |
Activated |
Deactivated |
Deactivated |
|
Position of E (relative to position of G) |
2- and/or 4- |
2- and/or 4- |
9. Qualitative Analysis Notes
[ppt. = precipitate]
(a) Reactions of aqueous cations
|
cation |
reaction with |
|
|
NaOH(aq) |
NH3(aq) |
|
|
aluminium, Al3+(aq) |
white ppt. soluble in excess |
white ppt. insoluble in excess |
|
ammonium, NH4+ (aq) |
ammonia produced on heating |
– |
|
barium, Ba2+(aq) |
no ppt. (if reagents are pure) |
no ppt. |
|
calcium, Ca2+(aq) |
white ppt. with high [Ca2+(aq)] |
no ppt. |
|
chromium(III), Cr3+(aq) |
grey-green ppt. soluble in excess giving dark green solution |
grey-green ppt. insoluble in excess |
|
copper(II), Cu2+(aq) |
pale blue ppt. insoluble in excess |
blue ppt. soluble in excess giving dark blue solution |
|
iron(II), Fe2+(aq) |
green ppt., turning brown on contact with air insoluble in excess |
green ppt., turning brown on contact with air insoluble in excess |
|
iron(III), Fe3+(aq) |
red-brown ppt. insoluble in excess |
red-brown ppt. insoluble in excess |
|
magnesium, Mg2+(aq) |
white ppt. insoluble in excess |
white ppt. insoluble in excess |
|
manganese(II), Mn2+(aq) |
off-white ppt., rapidly turning brown on contact with air insoluble in excess |
off-white ppt., rapidly turning brown on contact with air insoluble in excess |
|
zinc, Zn2+(aq) |
white ppt. soluble in excess |
white ppt. soluble in excess |
(b) Reactions of anions
|
anion |
reaction |
|
carbonate, CO32– |
CO2 liberated by dilute acids |
|
chloride, Cl–(aq) |
gives white ppt. with Ag+(aq) (soluble in NH3(aq)) |
|
bromide, Br–(aq) |
gives pale cream ppt. with Ag+(aq) (partially soluble in NH3(aq)) |
|
iodide, I–(aq) |
gives yellow ppt. with Ag+(aq) (insoluble in NH3(aq)) |
|
nitrate, NO3–(aq) |
NH3 liberated on heating with OH–(aq) and Al foil |
|
nitrite, NO2–(aq) |
NH3 liberated on heating with OH–(aq) and Al foil; NO liberated by dilute acids (colourless NO →(pale) brown NO2 in air) |
|
sulfate, SO42–(aq) |
gives white ppt. with Ba2+(aq) (insoluble in excess dilute strong acids) |
|
sulfite, SO32–(aq) |
SO2 liberated with dilute acids; gives white ppt. with Ba2+(aq) (soluble in dilute strong acids) |
(c) Tests for gases
|
gas |
test and test result |
|
ammonia, NH3 |
turns damp red litmus paper blue |
|
carbon dioxide, CO2 |
gives a white ppt. with limewater (ppt. dissolves with excess CO2) |
|
chlorine, Cl2 |
bleaches damp litmus paper |
|
hydrogen, H2 |
“pops” with a lighted splint |
|
oxygen, O2 |
relights a glowing splint |
|
sulfur dioxide, SO2 |
turns aqueous acidified potassium manganate(VII) from purple to colourless |
(d) Colour of halogens
|
halogen |
colour of element |
colour in aqueous solution |
colour in hexane |
|
chlorine, Cl2 |
greenish yellow gas |
pale yellow |
pale yellow |
|
bromine, Br2 |
reddish brown gas / liquid |
orange |
orange-red |
|
iodine, I2 |
black solid / purple gas |
brown |
purple |
(e) Tests for organic compounds (available for H2 Chemistry Syllabus 9476 Paper 4 Practical Exams only)
|
organic compounds |
reactions |
|
alkene |
decolourises orange Br2(aq) |
|
chloroalkane |
heat with NaOH(aq), |
|
bromoalkane |
heat with NaOH(aq), |
|
iodoalkane |
heat with NaOH(aq), |
|
alcohol |
• forms white fumes with solid PCl5 |
|
phenol |
decolourises orange Br2(aq) and forms a white ppt |
|
carbonyl compounds |
• gives orange ppt. with 2,4-dinitrophenylhydrazine |
|
carboxylic acid |
• CO2 liberated with Na2CO3(aq) |
|
phenylamine |
decolourises orange Br2(aq) and form a white ppt |
|
primary amide |
10. The Periodic Table of Elements
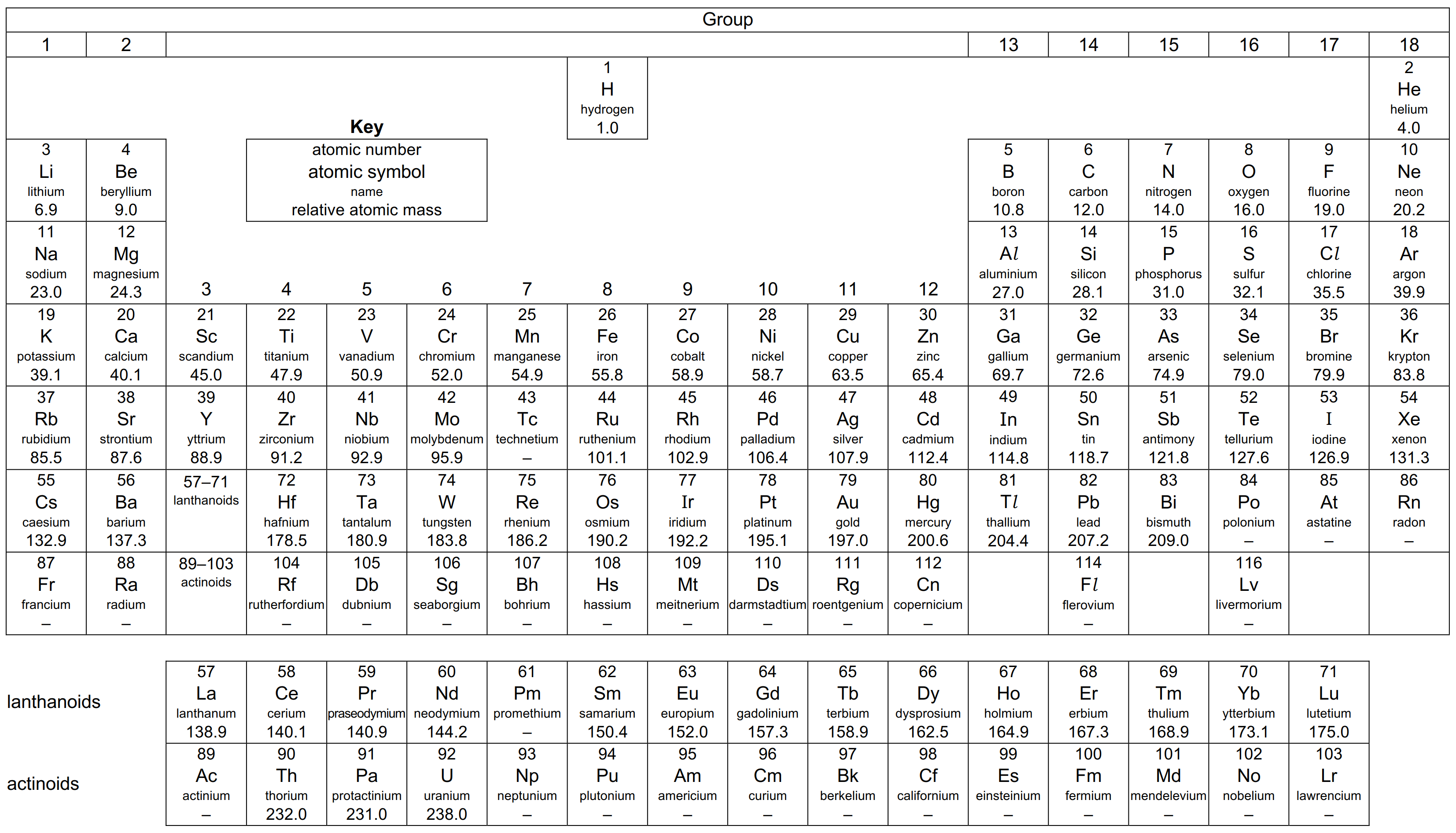
Click below for expandable version: